Accelerated Demand for Advanced Diagnostic Equipment Fuels North America Traumatic Brain Injury Diagnostics Equipment Market
According to the Medical Research Council 2022 report, 10 million people across the world sustain traumatic brain injury (TBI) annually. Among these, the male population was 1.5 times higher than females admitted to hospitals for a head injury. As per The Economist Intelligence Unit report, the global healthcare burden of TBI is estimated to be around US$ 400 billion annually. The most common form of ABI is TBI due to an accident or stroke.The Centers for Disease Control and Prevention (CDC) report revealed that an estimated 1.7 million TBI-related emergency department visits, hospitalizations, and deaths occur annually in the US, especially among adults aged 75 years and older as they are at high risk of falling due to problems with gait and balance. Also, road accidents are the leading cause of TBI-related deaths in the US and are highest among adults aged 20-24 years. Therefore, manufacturers are developing innovative products to diagnose TBI. In October 2023, bioMérieux announced Conformité Européenne (CE) marking for "VIDAS TBI (GFAP, UCH-L1)," a test intended to improve the assessment of patients suffering from mild traumatic brain injury (mTBI).
VIDAS TBI (GFAP, UCH-L1) test measures the concentration of glial fibrillary acidic protein (GFAP) and ubiquitin C-terminal hydrolase L1 (UCH-L1) - the two brain biomarkers released into the bloodstream starting from the first hour following a brain injury. It is an easy-to-interpret test providing a test window of up to 12 hours after injury, which can help in shortening total emergency department workup time.
North America Traumatic Brain Injury Diagnostics Equipment Market Overview
The accelerated demand for advanced diagnostic equipment and quick and effective diagnosis for TBI patients are the key driving factors behind the market development. However, adverse effects of contrast/medium agent is hampering the market growth. The Centers for Disease Control and Prevention (CDC) report revealed that an estimated 2.5 million people suffer from TBI annually in the US.According to the KNAPP & ROBERTS report, 1 in every 6 Americans live with TBI-related disability in the US alone, accounting for approx. 5.3 million. With the rising prevalence of TBI, the economic cost accounts for US$ 76.5 billion. Among US$ 76.5 billion, US$ 11.5 billion accounts for direct medical costs and nearly US$ 65 billion for indirect costs.
The leading causes of TBI include falls (45%), motor vehicle crashes (14.3%), assaults (10.7%), and unknown (19.0%). Further, companies are launching innovative products for the diagnosis of TBI. For instance, in August 2022, Abbott announced a new blood test for concussions to predict outcomes from brain injury and treatment interventions. The researchers used Abbott's "i-STAT TBI Plasma Test," the only FDA-cleared rapid test intended for concussion, and Abbott's core laboratory ARCHITECT instrument to measure two biomarkers in blood plasma associated with brain injury.
The i-STAT TBI Plasma test measures the level of biomarkers released in the bloodstream in response to the brain injury; the level of biomarkers assist in determining the need for a CT scan. Additionally, Sense Neuro Diagnostics announced clearance to conduct clinical trials for hemorrhage detection. The new trial approved by the FDA Division of Neurosurgical, Neurointerventional, and Neurodiagnostic Devices began in June 2023, including up to 300 patients at 30 US, Canada, and India sites. This noninvasive technology has a potential to collect 360 data points within 2.5 seconds to detect brain hemorrhage or stroke type, thereby helping quick response by physicians, emergency department personnels, neuro ICU teams, and military field hospitals assessing and monitoring TBI.
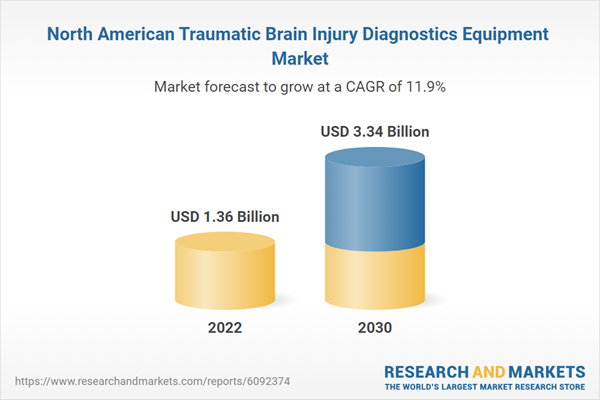
North America Traumatic Brain Injury Diagnostics Equipment Market Revenue and Forecast to 2030 (US$ Million)
North America Traumatic Brain Injury Diagnostics Equipment Market Segmentation
The North America traumatic brain injury diagnostics equipment market is categorized into techniques, device, end user, and country.- Based on techniques, the North America traumatic brain injury diagnostics equipment market is segmented into non-invasive, invasive, and combination techniques. The invasive segment held the largest North America traumatic brain injury diagnostics equipment market share in 2022. Furthermore, the non-invasive segment is subcategorized into electroencephalography (EEG), brain-computer interface (BCI), eye tracking and near-infrared spectroscopy (NIRS), magnetic resonance imaging (MRI), magnetoencephalography (MEG), transcranial magnetic stimulation (TMS), cerebral metabolic rate of oxygen (CMRO2), intracranial pressure, and others. Additionally, invasive segment is sub segmented into cerebral perfusion pressure (CPP) and pressure reactivity index (PRx), partial brain tissue oxygen pressure (PbtO2), cerebral microdialysis (CMD), intracranial pressure, and others.
- In terms of device, the North America traumatic brain injury diagnostics equipment market is bifurcated into imaging devices and monitoring devices. The imaging devices segment held a larger North America traumatic brain injury diagnostics equipment market share in 2022.
- By end user, the North America traumatic brain injury diagnostics equipment market is divided into hospitals and clinics, diagnostic centers, and others. The hospitals and clinics segment held the largest North America traumatic brain injury diagnostics equipment market share in 2022.
- By country, the North America traumatic brain injury diagnostics equipment market is segmented into the US, Canada, and Mexico. The US dominated the North America traumatic brain injury diagnostics equipment market share in 2022.
Reasons to Buy:
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the North America traumatic brain injury diagnostics equipment market.
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the North America traumatic brain injury diagnostics equipment market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth North America market trends and outlook coupled with the factors driving the North America traumatic brain injury diagnostics equipment market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution.
Table of Contents
Companies Mentioned
Some of the leading companies in the North America Traumatic Brain Injury Diagnostics Equipment Market include:- GE HealthCare Technologies Inc
- Elekta AB
- Integra LifeSciences Holdings Corp
- Natus Medical Inc
- Raumedic AG
- BrainScope Co Inc
- Luciole Medical AG
- Soterix Medical Inc
- Medtronic Plc
- Vivonics Inc
- NanoDx Inc
- Compumedics Ltd
- Sense Diagnostics Inc
- NeuraSignal Inc
- Neurovigil Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 204 |
| Published | March 2025 |
| Forecast Period | 2022 - 2030 |
| Estimated Market Value ( USD | $ 1.36 Billion |
| Forecasted Market Value ( USD | $ 3.34 Billion |
| Compound Annual Growth Rate | 11.9% |
| Regions Covered | North America |
| No. of Companies Mentioned | 15 |