Increasing Focus on Patient-Centered Care Drives Europe Electronic Patient-Reported Outcomes (ePROS) Market
The growing emphasis on patient-centered care fuels the growth of the electronic patient-reported outcomes (ePROS) market. Tools that gather patient feedback in real-time are in high demand owing to the shift toward prioritizing patient experiences, outcomes, and preferences. The real-time tracking of patient-reported health outcomes is made possible by ePROs as healthcare becomes more personalized and value-based. Better health outcomes are supported by ensuring treatments are tailored to each patient's needs. The use of digital tools such as ePROs has increased as the focus of healthcare facilities shifts to patient experience and satisfaction.With the help of this technology, patients can have less stress and receive continuous feedback by giving healthcare providers access to precise data. This change drives innovation in digital health technologies, supporting the integration of ePROs into medical procedures. For instance, healthcare providers can easily monitor patient outcomes owing to the growing integration of ePRO tools with electronic health record (EHR) systems. This facilitates the alignment of clinical decisions with the preferences and experiences that the patient has reported. Therefore, a greater understanding of patient-centered care propels the use of ePROs.
Europe Electronic Patient-Reported Outcomes (ePROS) Market Overview
According to Clinical Trials Arena, Germany held ~3.9% of the total clinical trials carried out worldwide in 2021. Furthermore, there is a massive patient pool and a high demand for quality healthcare in Germany. Coordinating centers for clinical trials were set up as part of a new funding program under the Federal Ministry of Education and Research to further encourage academic clinical research. The Federal Institute for Drugs and Medical Devices or the Paul-Ehrlich Institute approves clinical trials in Germany, depending on the product to be investigated. Thus, Germany's clinical trial approval processes are standardized, transparent, reliable, and approved for relatively short study startup timelines.To further enhance the clinical trial process and evaluation, the increased use of digital technologies such as ePROs plays an important role. In addition, the development of digital health technology and integration of artificial intelligence is also rising in the country. According to the Germany Trade and Invest report, the healthcare industry in Germany is undergoing massive digitalization. By 2025, the digital health market in Germany is projected to be worth US$ 63 billion. Therefore, the rise in clinical trials and an upsurge in the adoption of digital health technologies are expected to fuel the electronic related-patient outcomes (ePROS) market in the coming years.
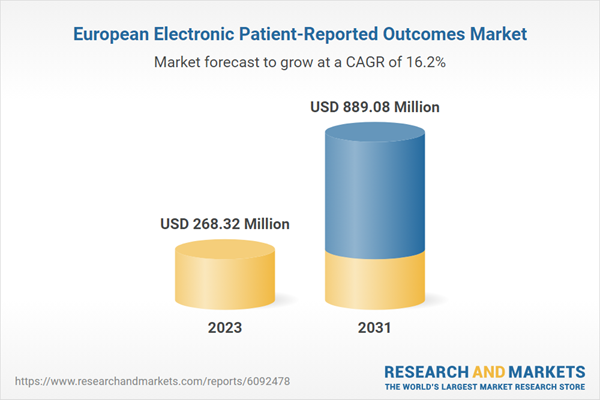
Europe Electronic Patient-Reported Outcomes (ePROS) Market Revenue and Forecast to 2031 (US$ Million)
Europe Electronic Patient-Reported Outcomes (ePROS) Market Segmentation
The Europe electronic patient-reported outcomes (ePROS) market is categorized into delivery mode, application, end user, and country.By delivery mode, the Europe electronic patient-reported outcomes (ePROS) market is bifurcated into cloud based and on-premises. The cloud based segment held a larger share of the Europe electronic patient-reported outcomes (ePROS) market share in 2023.
- In terms of application, the Europe electronic patient-reported outcomes (ePROS) market is segmented into oncology, respiratory, and others. The oncology segment held the largest share of the Europe electronic patient-reported outcomes (ePROS) market share in 2023.
- By end user, the Europe electronic patient-reported outcomes (ePROS) market is segmented into contract research organizations (CROs), pharmaceutical companies, and others. The pharmaceutical companies segment held the largest share of the Europe electronic patient-reported outcomes (ePROS) market share in 2023.
- Based on country, the Europe electronic patient-reported outcomes (ePROS) market is segmented into the UK, Germany, France, Italy, Spain, and the Rest of Europe. Germany segment held the largest share of Europe electronic patient-reported outcomes (ePROS) market in 2023.
Reasons to Buy:
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the Europe electronic patient-reported outcomes (ePROS) market.
- Highlights key business priorities to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the Europe electronic patient-reported outcomes (ePROS) market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth Europe market trends and outlook coupled with the factors driving the Europe electronic patient-reported outcomes (ePROS) market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution.
Table of Contents
Companies Mentioned
Some of the leading companies in the Europe Electronic Patient-Reported Outcomes (ePROS) Market include:- Assistek
- Buddy Healthcare Ltd Oy
- Castor
- Clinical Ink Inc
- Crucial Data Solutions
- Curebase
- Medable Inc
- Medidata Solutions
- Medrio
- OpenClinica LLC
- PatientIQ
- Signant Health
- Veeva Systems Inc
- Y-Prime LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 91 |
| Published | March 2025 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 268.32 Million |
| Forecasted Market Value ( USD | $ 889.08 Million |
| Compound Annual Growth Rate | 16.2% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 14 |