Emerging Applications of Electronic Patient-Reported Outcomes (ePROS) Fuel North America Electronic Patient-Reported Outcomes (ePROS) Market
The emerging applications of electronic patient-reported outcomes (ePRO) drive the market. The new uses of ePRO systems in decentralized clinical trials, remote patient monitoring, and other areas are stimulating innovation and extending the use of patient-reported outcomes in various healthcare, research, and clinical settings. The adoption of ePRO systems is driven by decentralized clinical trials (DCTs) growth. Patients can participate in DCTs remotely from the comfort of their homes, and ePRO tools are widely used for collecting patient-reported data immediately.The transition to virtual trials improves patient recruitment and retention, lowers the need for in-person visits, and increases trial efficiency of various diseases, including rare diseases, across geographically dispersed populations. In addition, the players are making strategic developments to meet the customers' needs. For instance, in June 2023, uMotif, a clinical trial technology company, and Syneos Health, an integrated biopharmaceutical solution company, announced a strategic partnership. By developing a more effective, end-to-end digital platform with electronic solid clinical outcome assessment (eCOA) and electronic patient-reported outcomes (ePRO) capabilities - this partnership helped accelerate clinical trials and expedite the delivery of new therapies to patients.
ePRO systems are being used to monitor acute conditions such as post-operative recovery and chronic conditions, including diabetes and hypertension, as healthcare increasingly moves toward telemedicine and remote patient monitoring (RPM). To help healthcare providers make regular interventions and changes to care plans, patients can report symptoms, medication adherence, and treatment responses from the comfort of their own homes. This approach lowers the rate of hospital readmissions, enhances chronic illness management, and improves patient satisfaction by offering ongoing, individualized care without requiring frequent in-person visits.
The adoption of ePRO systems is propelled by the growing use of wearables, including smartwatches, fitness trackers, and Internet of Things (IoT) devices in the healthcare industry. Predictive healthcare interventions can be made possible by combining objective data from wearables with subjective patient-reported outcomes. This results in better condition monitoring and proactive condition management. Pharmaceutical companies are utilizing ePRO systems to monitor adverse events in real-world settings, helping them gain traction in pharmacovigilance and post-market surveillance, thereby ensuring long-term drug safety and efficacy while adhering to regulatory requirements.
North America Electronic Patient-Reported Outcomes (ePROS) Market Overview
The North America electronic patient-reported outcomes (ePROS) market is segmented into the US, Canada, and Mexico. The US is one of the most developed countries in North America. The country accounts for the major market share owing to the increasing use of digital health technologies, which helps provide patient-centered care. This involves integrating healthcare services with software and hardware, which can be managed and monitored with the help of apps, wearable devices, and other devices. The increasing focus on chronic disease management has increased the demand for ePROs as they allow continuous assessment and timely interventions.As per the article published by the American Society of Clinical Oncology in June 2023, in the US, ePRO inclusion in the Centers for Medicare and Medicaid Innovation's alternative payment model pilot, the Enhancing Oncology Model, is a sign that ePRO implementation should be a mainstay of high-quality and transformational cancer care and is likely to foster broader adoption. The surging number of clinical trials in the country is expected to increase the demand for ePROs. Market players are taking electronic related patient outcomes (ePROS) market strategic initiatives such as product launches, product approvals, and collaborations, which are further expected to amplify the market growth.
For instance, in June 2024, Evidation launched Migraine Smart, which is a health engagement experience and symptom tracking program on the Evidation app that harnesses survey data, electronic patient reported outcomes (ePROs), wearable data, and evidence-based content to make it possible for individuals to better understand and manage their migraines. Therefore, an increase in digital health technologies, a surge in demand for patient-centered care, and increase in strategic initiatives by market players are expected to fuel the growth of the North America ePROs Market in the coming years.
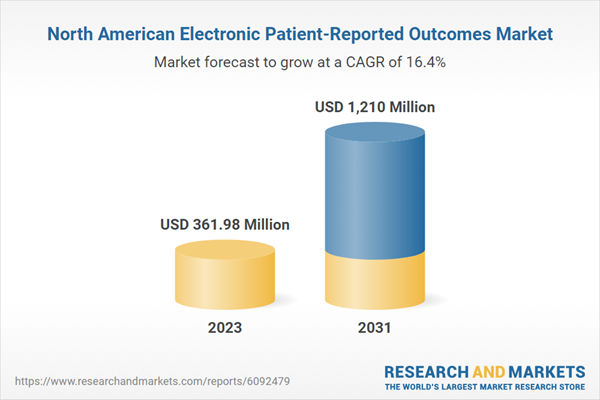
North America Electronic Patient-Reported Outcomes (ePROS) Market Revenue and Forecast to 2031 (US$ Million)
North America Electronic Patient-Reported Outcomes (ePROS) Market Segmentation
The North America electronic patient-reported outcomes (ePROS) market is categorized into delivery mode, application, end user, and country.By delivery mode, the North America electronic patient-reported outcomes (ePROS) market is segmented into cloud based and on-premises. The cloud based segment held a larger share of the North America electronic patient-reported outcomes (ePROS) market share in 2023.
- In terms of application, the North America electronic patient-reported outcomes (ePROS) market is segmented into oncology, respiratory, and others. The oncology segment held the largest share of the North America electronic patient-reported outcomes (ePROS) market share in 2023.
- By end user, the North America electronic patient-reported outcomes (ePROS) market is segmented into contract research organizations (CROs), pharmaceutical companies, and others. The pharmaceutical companies segment held the largest share of the North America electronic patient-reported outcomes (ePROS) market share in 2023.
- Based on country, the North America electronic patient-reported outcomes (ePROS) market is segmented into the US, Canada, and Mexico. The US segment held the largest share of North America electronic patient-reported outcomes (ePROS) market in 2023.
Reasons to Buy:
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the North America electronic patient-reported outcomes (ePROS) market.
- Highlights key business priorities to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the North America electronic patient-reported outcomes (ePROS) market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth North America market trends and outlook coupled with the factors driving the North America electronic patient-reported outcomes (ePROS) market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution.
Table of Contents
Companies Mentioned
Some of the leading companies in the North America Electronic Patient-Reported Outcomes (ePROS) Market include:- Assistek
- Buddy Healthcare Ltd Oy
- Castor
- Clinical Ink Inc
- Crucial Data Solutions
- Curebase
- Medable Inc
- Medidata Solutions
- Medrio
- OpenClinica LLC
- PatientIQ
- Signant Health
- Veeva Systems Inc
- Y-Prime LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 82 |
| Published | March 2025 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 361.98 Million |
| Forecasted Market Value ( USD | $ 1210 Million |
| Compound Annual Growth Rate | 16.4% |
| Regions Covered | North America |
| No. of Companies Mentioned | 14 |