Global Nasal Vaccines Market - Key Trends & Drivers Summarized
Why Are Nasal Vaccines Gaining Momentum in Immunization Strategies?
Nasal vaccines are increasingly recognized as a transformative advancement in vaccine delivery systems, offering needle-free, mucosal-based immunization that mimics natural infection pathways. Unlike injectable vaccines that stimulate systemic immunity, nasal vaccines target the mucosal surfaces - the primary entry points for many respiratory pathogens - thereby inducing both systemic and local immunity. This dual response not only improves protective efficacy but also reduces viral shedding and transmission, making nasal vaccines especially valuable during infectious disease outbreaks.Their ease of administration, particularly in pediatric and needle-phobic populations, is reshaping mass vaccination logistics. Nasal sprays do not require trained personnel or syringes, simplifying distribution in low-resource settings. These features are driving increasing global interest, especially for diseases like influenza, COVID-19, and respiratory syncytial virus (RSV), where rapid, large-scale immunization is often critical. The potential for self-administration and the elimination of biohazardous waste associated with needles further underscore the public health value of this platform.
What Scientific Advances Are Enhancing the Efficacy and Stability of Nasal Vaccines?
Significant innovations in formulation science and delivery technology are boosting the development of nasal vaccines. One major advancement is the use of novel adjuvants and mucoadhesive carriers that enhance antigen retention in the nasal cavity, leading to stronger and longer-lasting immune responses. Liposomes, nanoemulsions, and polymer-based delivery systems are being engineered to protect antigens from degradation while promoting uptake by nasal-associated lymphoid tissue (NALT), the immune inductive site for mucosal immunity.Thermostability is another area of progress, with researchers developing dry powder nasal vaccines that can be stored at ambient temperatures, bypassing the need for cold-chain logistics. This attribute is especially crucial for deployment in remote or underdeveloped regions. Additionally, recombinant protein subunits and viral vectors are being tailored for intranasal use, offering broader antigen expression profiles and multivalent targeting. Collectively, these advancements are making nasal vaccines more robust, versatile, and compatible with a wider range of pathogens.
How Are End-Use Applications and Public Health Strategies Expanding?
The end-use landscape for nasal vaccines is evolving rapidly, with increasing integration into national immunization schedules, school-based vaccine programs, and pandemic preparedness strategies. Pediatric and geriatric populations are emerging as key target groups due to their high susceptibility to respiratory diseases and the challenges they often face with needle-based vaccinations. Moreover, the ability to administer nasal vaccines without the need for intravenous access makes them highly suitable for use during emergencies and outbreaks in field conditions.In addition to infectious diseases, research is expanding into nasal vaccines for non-communicable applications such as allergies, Alzheimer's disease, and cancer immunotherapy. The nasal route provides direct access to the central nervous system via the olfactory bulb, making it an attractive portal for neurological and systemic immune modulation. Government and private-sector collaborations are increasingly exploring nasal immunization not only as a medical solution but as a strategic tool for boosting vaccine acceptance, reducing healthcare system burden, and accelerating outbreak response.
What Factors Are Driving Growth in the Nasal Vaccines Market?
The growth in the nasal vaccines market is driven by several factors tied to advancements in delivery platforms, expansion in target disease profiles, and systemic shifts in immunization infrastructure. One of the primary drivers is the rising demand for needle-free vaccine options that improve patient compliance, particularly among children, the elderly, and needle-averse individuals. This demand is further amplified in mass immunization settings where ease of administration and minimal training requirements reduce logistical complexity.End-use diversification across public health, travel medicine, pediatric care, and emergency outbreak response is expanding the commercial viability of nasal vaccines. The increased incidence of respiratory illnesses and global preparedness efforts against pandemic threats are driving demand for rapid, scalable, and non-invasive vaccine delivery solutions. Additionally, supportive regulatory pathways and funding initiatives focused on next-generation vaccine platforms are catalyzing R&D investments and accelerating clinical trials.
Technological improvements in formulation stability, cold-chain independence, and multi-antigenic capability are also enhancing the value proposition of nasal vaccines. These attributes are making them particularly attractive for low-resource settings and global vaccination initiatives where traditional injectable vaccines face logistical challenges. As stakeholders across healthcare, biotechnology, and global health policy align around the benefits of mucosal immunization, the nasal vaccines market is poised for robust growth and significant public health impact.
Report Scope
The report analyzes the Nasal Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Vaccine Type (Live Attenuated Vaccines, Inactivated Vaccines, Subunit, Recombinant, Conjugate Vaccines, Other Vaccine Types); Distribution Channel (Public, Private).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Live Attenuated Vaccines segment, which is expected to reach US$190.5 Million by 2030 with a CAGR of a 9.3%. The Inactivated Vaccines segment is also set to grow at 8.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $119.7 Million in 2024, and China, forecasted to grow at an impressive 11.5% CAGR to reach $142.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Nasal Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Nasal Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Nasal Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Absen, AOTO Electronics, Barco, Christie Digital Systems, Daktronics and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Nasal Vaccines market report include:
- Altimmune, Inc.
- AstraZeneca
- Bharat Biotech
- BioDiem Ltd
- BioNTech SE
- BlueWillow Biologics
- Changchun BCHT Biotechnology Co.
- Codagenix Inc.
- CyanVac LLC
- FluGen Inc.
- Gamma Vaccines Pty Ltd.
- GlaxoSmithKline plc
- Intravacc
- Meissa Vaccines Inc.
- Ocugen, Inc.
- Pfizer Inc.
- Razi Vaccine and Serum Research Institute
- Sanofi
- Serum Institute of India Pvt. Ltd.
- Vaxart, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Altimmune, Inc.
- AstraZeneca
- Bharat Biotech
- BioDiem Ltd
- BioNTech SE
- BlueWillow Biologics
- Changchun BCHT Biotechnology Co.
- Codagenix Inc.
- CyanVac LLC
- FluGen Inc.
- Gamma Vaccines Pty Ltd.
- GlaxoSmithKline plc
- Intravacc
- Meissa Vaccines Inc.
- Ocugen, Inc.
- Pfizer Inc.
- Razi Vaccine and Serum Research Institute
- Sanofi
- Serum Institute of India Pvt. Ltd.
- Vaxart, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 220 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
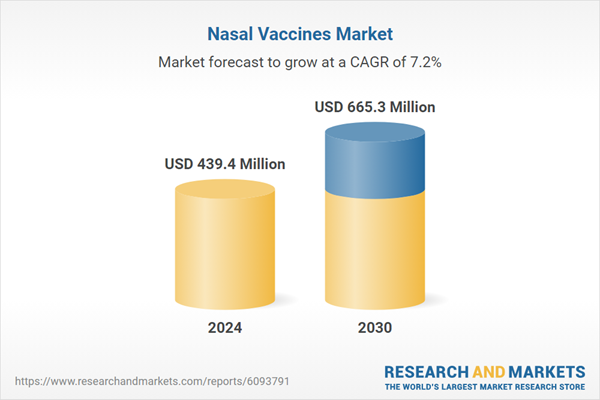
| Estimated Market Value ( USD | $ 439.4 Million |
| Forecasted Market Value ( USD | $ 665.3 Million |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |