Global Nerve Monitoring Devices Market - Key Trends & Drivers Summarized
Why Are Nerve Monitoring Devices Gaining Critical Importance in Surgical Settings?
Nerve monitoring devices are becoming indispensable tools in modern surgical procedures, where preserving neural function is essential to improving patient outcomes and reducing postoperative complications. These systems are primarily used during complex surgeries involving the spine, head and neck, or peripheral nervous system, where there is a high risk of inadvertent nerve damage. Real-time intraoperative neuromonitoring (IONM) allows surgeons to identify and protect motor and sensory nerves by providing immediate feedback on nerve function during dissection and tissue manipulation.The rising complexity and frequency of surgeries in neurosurgery, otolaryngology, orthopedic, and cardiovascular fields are driving the clinical need for accurate, responsive, and user-friendly nerve monitoring tools. With the increasing adoption of minimally invasive and robot-assisted surgeries, the margin for error has narrowed considerably. Nerve monitoring devices address this challenge by enhancing precision and reducing the likelihood of neurological deficits such as vocal cord paralysis, limb weakness, or incontinence. This growing focus on function-preserving surgeries has firmly positioned nerve monitoring systems as standard protocol in high-risk procedures.
What Technological Innovations Are Enhancing Accuracy and Clinical Utility?
The nerve monitoring landscape is undergoing a technological transformation, marked by improvements in signal detection, user interface design, and system integration. Electromyography (EMG)-based devices, somatosensory evoked potential (SSEP) systems, and motor evoked potential (MEP) technologies are becoming more refined in their ability to differentiate real neural signals from surgical artifacts. Advanced systems now feature noise-reduction algorithms, customizable alerts, and multi-channel monitoring capabilities that allow for more precise nerve mapping and safer surgical navigation.Wireless probes, touchscreen interfaces, and AI-enabled interpretation modules are further simplifying intraoperative use while enhancing decision-making accuracy. Integration with surgical navigation systems and robotic-assisted platforms is creating seamless workflows where nerve monitoring is not an auxiliary function but a core component of surgical planning and execution. Additionally, compact and portable nerve monitoring units are enabling broader deployment in outpatient and ambulatory surgical centers, where space and resource constraints previously limited usage. These innovations are not only improving safety but also expanding the scope of procedures where nerve monitoring can be effectively applied.
How Are End-Use Patterns Shifting Across Healthcare Facilities?
The use of nerve monitoring devices is rapidly expanding beyond academic hospitals and tertiary care centers into community hospitals and outpatient surgical facilities. This diffusion is being driven by the standardization of best practices in nerve protection, as well as increased surgeon familiarity with neuromonitoring protocols. High-volume procedures such as thyroidectomy, parotidectomy, spinal decompression, and orthopedic joint repair are now routinely incorporating intraoperative nerve monitoring as part of risk mitigation and quality assurance efforts.Pediatric and geriatric surgeries, where neural structures may be more vulnerable, are also witnessing increased use of neuromonitoring tools. Moreover, surgical training programs are beginning to incorporate nerve monitoring into curricula, which is fostering a new generation of surgeons who view these systems as essential rather than optional. Health systems aiming to minimize litigation and enhance postoperative recovery metrics are adopting nerve monitoring devices as part of bundled surgical care packages. The convergence of safety, education, and efficiency objectives is accelerating institutional adoption across a broader range of care settings.
What Factors Are Driving Growth in the Nerve Monitoring Devices Market?
The growth in the nerve monitoring devices market is driven by several key factors related to surgical demand, technology integration, and evolving care standards. A primary driver is the global increase in complex surgical procedures - especially spine, cranial, ENT, and orthopedic operations - where the risk of nerve injury necessitates real-time functional guidance. As patient safety becomes a core metric in surgical outcomes, the use of IONM devices is transitioning from discretionary to essential.End-use expansion across hospitals, ambulatory surgical centers, and specialized clinics is further boosting market penetration. The rapid adoption of minimally invasive and robotic-assisted surgeries is creating a need for nerve monitoring systems that can integrate seamlessly with modern operating room technologies. Additionally, healthcare regulations emphasizing postoperative quality metrics and complication reduction are encouraging providers to adopt neuromonitoring tools as part of standard surgical workflows.
Ongoing advancements in monitoring modalities, data interpretation, and device ergonomics are also increasing clinician confidence and usability. Reimbursement improvements and rising awareness among healthcare professionals about the medico-legal advantages of documented nerve monitoring are reinforcing the device's value proposition. Collectively, these factors are propelling strong market growth, making nerve monitoring devices a core pillar of future-ready surgical care across the globe.
Report Scope
The report analyzes the Nerve Monitoring Devices market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Technology (Electroencephalogram, Electromyography, Electrocorticography, Evoked Potential); Product (Monitor, Electrodes, Ancillary Products).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Electroencephalogram segment, which is expected to reach US$567.3 Million by 2030 with a CAGR of a 2.8%. The Electromyography segment is also set to grow at 2.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $351 Million in 2024, and China, forecasted to grow at an impressive 6.1% CAGR to reach $307.9 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Nerve Monitoring Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Nerve Monitoring Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Nerve Monitoring Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Agenus Inc., BioNTech SE, Candel Therapeutics, Genelux Corporation, Geneos Therapeutics and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Nerve Monitoring Devices market report include:
- Advanced Brain Monitoring
- Cadwell Industries
- Checkpoint Surgical
- Compumedics Limited
- Dr. Langer Medical GmbH
- Electrical Geodesics Inc.
- EMS Biomedical
- Erbe Elektromedizin GmbH
- GE Healthcare
- Inomed Medizintechnik GmbH
- Langer Medical GmbH
- Magstim
- Medtronic
- Natus Medical Incorporated
- NeuroVision Imaging, Inc.
- Neurovision Medical Products
- Neuspera Medical
- Nihon Kohden Corporation
- NuVasive, Inc.
- Xavant Technology
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Advanced Brain Monitoring
- Cadwell Industries
- Checkpoint Surgical
- Compumedics Limited
- Dr. Langer Medical GmbH
- Electrical Geodesics Inc.
- EMS Biomedical
- Erbe Elektromedizin GmbH
- GE Healthcare
- Inomed Medizintechnik GmbH
- Langer Medical GmbH
- Magstim
- Medtronic
- Natus Medical Incorporated
- NeuroVision Imaging, Inc.
- Neurovision Medical Products
- Neuspera Medical
- Nihon Kohden Corporation
- NuVasive, Inc.
- Xavant Technology
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 274 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
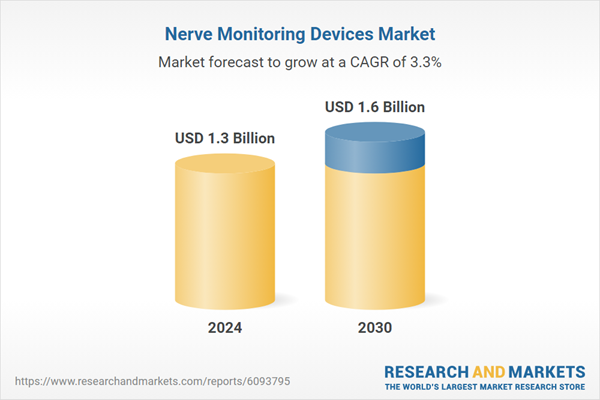
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 1.6 Billion |
| Compound Annual Growth Rate | 3.3% |
| Regions Covered | Global |