Global Cancer Antibody Drug Conjugates (ADCs) Market - Key Trends & Drivers Summarized
Why Are Cancer Antibody Drug Conjugates Gaining Strategic Importance Across Oncology Therapeutics, Targeted Drug Delivery, and Precision Medicine Pipelines?
Antibody drug conjugates (ADCs) are emerging as a transformative class of oncology therapeutics, combining the tumor-targeting specificity of monoclonal antibodies with the cytotoxic potency of chemotherapeutic agents. Designed to selectively deliver cancer-killing payloads to malignant cells while sparing healthy tissue, ADCs offer a powerful alternative to traditional chemotherapy and systemic biologics. Their ability to enhance therapeutic efficacy while reducing systemic toxicity is positioning ADCs at the forefront of precision oncology strategies for solid tumors and hematologic malignancies.Rising cancer prevalence, increasing resistance to conventional therapies, and demand for next-generation targeted treatments are accelerating ADC adoption. Pharma pipelines are expanding rapidly, supported by technological improvements in linker chemistry, site-specific conjugation, and payload diversification. With several high-profile FDA and EMA approvals in breast, bladder, and hematologic cancers, ADCs are gaining traction not only in late-stage treatment but also in earlier lines of therapy and combination regimens - reshaping clinical practice in oncology.
How Are Payload Engineering, Conjugation Technology, and Biomarker Integration Advancing the Clinical and Commercial Potential of ADCs?
Breakthroughs in ADC design are driving a new wave of high-performance molecules with improved safety profiles and broader clinical applicability. Innovations in linker stability and cleavability are enabling more precise payload release within tumor cells while minimizing premature off-target effects. Advanced conjugation technologies - such as site-specific or enzymatic conjugation - are yielding homogenous drug-to-antibody ratios (DAR), enhancing batch consistency and therapeutic predictability.The range of payloads is expanding beyond traditional microtubule inhibitors to include DNA-damaging agents, topoisomerase inhibitors, and immune-modulatory compounds. Dual payload ADCs and bispecific antibody formats are under active investigation to address tumor heterogeneity and drug resistance. Biomarker-driven patient selection and companion diagnostic co-development are further strengthening ADC clinical positioning, particularly in HER2-positive, TROP2-expressing, and CD30-targeted cancers. These precision-guided approaches are optimizing therapeutic windows, improving outcomes, and opening new indications for ADC application.
Which Cancer Types, Geographic Markets, and Biopharma Stakeholders Are Driving ADC Market Expansion?
Breast cancer, particularly HER2-positive subtypes, remains a leading indication for commercialized ADCs, with bladder, non-small cell lung cancer (NSCLC), and hematologic cancers such as Hodgkin lymphoma and multiple myeloma following closely. Pipeline activity is expanding into ovarian, gastric, prostate, and pancreatic cancers, where unmet need and biomarker stratification offer significant opportunity.North America leads ADC market activity, driven by strong clinical infrastructure, regulatory acceleration, and concentrated biopharma innovation. Europe maintains robust clinical trial momentum and uptake of approved ADCs under its oncology reimbursement frameworks. Asia-Pacific is witnessing rapid growth, particularly in China and Japan, where domestic R&D pipelines, cross-border licensing, and oncology-focused biotech ecosystems are gaining scale. Global demand is further supported by rising cancer incidence, expanded biomarker testing, and inclusion of ADCs in national treatment guidelines.
The market is being shaped by a mix of multinational pharmaceutical companies, oncology-focused biotechs, and CDMO partners specializing in ADC manufacturing. Licensing partnerships, M&A activity, and co-commercialization agreements are central to scaling global reach and accelerating time to market. CDMOs with cytotoxic handling capabilities and linker-payload expertise are emerging as critical enablers of manufacturing agility and regulatory compliance.
What Are the Factors Driving Growth in the Cancer Antibody Drug Conjugates Market?
The cancer ADC market is expanding rapidly as biopharma companies invest in more precise, potent, and patient-specific oncology therapies. ADCs are increasingly viewed as cornerstone assets in oncology portfolios, offering differentiated mechanisms of action and versatile application across tumor types and disease stages.Key growth drivers include expanding clinical validation across solid and liquid tumors, continuous innovation in linker and payload technology, regulatory support for accelerated approvals, rising demand for biomarker-driven therapies, and increasing payer acceptance based on survival benefit and reduced toxicity. The shift toward combination regimens and earlier-line therapies is also fueling long-term market sustainability.
As the convergence of antibody engineering, cytotoxic chemistry, and genomic targeting redefines cancer treatment, could antibody drug conjugates become the next dominant therapeutic modality anchoring the future of personalized oncology care?
Report Scope
The report analyzes the Cancer Antibody Drug Conjugates market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Kadcyla, Enhertu, Adcetris, Padcev, Trodelvy, Polivy, Other Products); Technology (Cleavable, Non-Cleavable); Application (Blood Cancer, Breast Cancer, Ovary Cancer, Lung Cancer, Skin Cancer, Brain Tumor, Other Applications); End-Use (Hospitals, Specialty Clinics, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Kadcyla segment, which is expected to reach US$5.7 Billion by 2030 with a CAGR of a 17.6%. The Enhertu segment is also set to grow at 12.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.3 Billion in 2024, and China, forecasted to grow at an impressive 20.3% CAGR to reach $4.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Cancer Antibody Drug Conjugates Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Cancer Antibody Drug Conjugates Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Cancer Antibody Drug Conjugates Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Anker Innovations, Bluetti Power Inc., Crave, Duracell Inc., EcoFlow and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Cancer Antibody Drug Conjugates market report include:
- AbbVie Inc.
- ADC Therapeutics SA
- Ambrx Inc.
- Astellas Pharma Inc.
- AstraZeneca plc
- Bayer AG
- Daiichi Sankyo Company, Limited
- F. Hoffmann-La Roche Ltd.
- Genmab A/S
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- ImmunoGen, Inc.
- Johnson & Johnson
- Merck KGaA
- Mersana Therapeutics, Inc.
- Pfizer Inc.
- RemeGen Co., Ltd.
- Samsung Biologics Co., Ltd.
- Seagen Inc.
- Sutro Biopharma, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- ADC Therapeutics SA
- Ambrx Inc.
- Astellas Pharma Inc.
- AstraZeneca plc
- Bayer AG
- Daiichi Sankyo Company, Limited
- F. Hoffmann-La Roche Ltd.
- Genmab A/S
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- ImmunoGen, Inc.
- Johnson & Johnson
- Merck KGaA
- Mersana Therapeutics, Inc.
- Pfizer Inc.
- RemeGen Co., Ltd.
- Samsung Biologics Co., Ltd.
- Seagen Inc.
- Sutro Biopharma, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 482 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
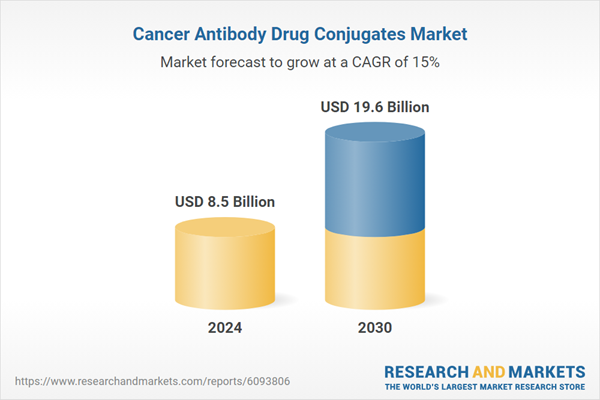
| Estimated Market Value ( USD | $ 8.5 Billion |
| Forecasted Market Value ( USD | $ 19.6 Billion |
| Compound Annual Growth Rate | 15.0% |
| Regions Covered | Global |