Global Chickenpox Vaccines Market - Key Trends & Drivers Summarized
Why Are Chickenpox Vaccines Central to Pediatric Immunization, Public Health Planning, and Outbreak Prevention?
Chickenpox vaccines have become a critical tool in global immunization strategies aimed at reducing the incidence of varicella-zoster virus (VZV) infections, particularly among children. Characterized by high transmissibility and the potential for severe complications in vulnerable populations, chickenpox poses a significant public health burden in regions lacking routine immunization programs. As governments increasingly adopt universal childhood vaccination policies, the chickenpox vaccine is gaining prominence in both public and private healthcare systems for its role in preventing illness, reducing school absenteeism, and minimizing healthcare resource utilization.Beyond childhood protection, vaccination is essential in immunocompromised individuals, non-immune adults, and healthcare workers who face elevated risks of exposure. As global mobility and urbanization accelerate, vaccine-driven immunity becomes key in preventing community-wide outbreaks, managing disease containment in institutions, and lowering the long-term healthcare impact of varicella-related complications such as bacterial superinfections or neurological sequelae.
How Are Vaccine Formulations, Cold Chain Infrastructure, and Coverage Strategies Evolving to Expand Impact?
Chickenpox vaccines, typically live attenuated virus formulations, have demonstrated high efficacy in preventing moderate to severe disease, with two-dose regimens significantly increasing long-term immunity. Research and development are ongoing to enhance vaccine stability, improve immunogenicity, and reduce adverse reaction profiles, especially in pediatric and immunocompromised subgroups. Combination vaccines that include chickenpox along with measles, mumps, and rubella (MMRV) are streamlining immunization schedules and improving compliance.Advancements in cold chain logistics, temperature-stable formulations, and vaccine vial monitors are enabling broader reach, particularly in remote and resource-limited regions. National immunization programs are increasingly integrating chickenpox vaccination into early childhood schedules, often with public funding or donor support to achieve herd immunity targets. School-entry mandates, outbreak response initiatives, and adult catch-up campaigns are further boosting coverage and awareness.
Digital health tools, immunization tracking platforms, and AI-driven demand forecasting are also being leveraged to improve vaccine delivery efficiency, monitor adverse events, and strengthen supply chain transparency.
Which End-Use Settings and Regional Health Policies Are Driving Uptake of Chickenpox Vaccination?
Primary delivery points include pediatric clinics, public health centers, school-based health programs, and travel medicine clinics. Mass vaccination is most prominent in countries where varicella is classified as a notifiable disease and where the vaccine is included in national immunization schedules. Private pediatric practices and urgent care centers in high-income countries also contribute significantly to adult catch-up and elective immunization volumes.North America and several parts of Europe have achieved near-universal childhood vaccination coverage, resulting in sharp declines in both disease incidence and hospitalization rates. Asia-Pacific is experiencing a mixed landscape, with countries like Japan and South Korea offering routine immunization, while others are gradually scaling up access through tiered regional programs. Latin America and the Middle East are expanding coverage through school-based programs and partnerships with global health agencies. In Africa, where varicella surveillance remains limited, uptake is growing in urban areas but is still in early stages across rural and underserved communities.
What Are the Factors Driving Growth in the Chickenpox Vaccines Market?
The market for chickenpox vaccines is expanding alongside public health efforts to control communicable diseases through early immunization. As global health agencies target disease eradication and long-term burden reduction, chickenpox vaccination is increasingly viewed as a preventive investment with measurable health system benefits.Core growth drivers include increasing childhood vaccination rates, growing awareness of varicella-related complications, integration of combination vaccines, expansion of immunization infrastructure, and strong policy support from national and international health bodies. Emerging disease surveillance frameworks and pandemic-resilient immunization campaigns are further reinforcing the need for robust, scalable vaccine delivery systems.
As global health systems strengthen their focus on early prevention, could chickenpox vaccines become a foundational pillar of universal immunization strategies across all income and risk segments?
Report Scope
The report analyzes the Chickenpox Vaccines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Monovalent Varicella Vaccine, Combination Varicella Vaccine); Application (Mumps, Measles, Rubella, Varicella Immunization, Herpes Zoster, Immunization, Chickenpox Vaccination); End-Use (Hospitals, Clinics, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Monovalent Varicella Vaccine segment, which is expected to reach US$2.5 Billion by 2030 with a CAGR of a 3.2%. The Combination Varicella Vaccine segment is also set to grow at 5.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $866.9 Million in 2024, and China, forecasted to grow at an impressive 7% CAGR to reach $802.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Chickenpox Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Chickenpox Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Chickenpox Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ACH Food Companies Inc., Ajinomoto Co., Inc., BASF SE, Cargill, Incorporated, Carnad and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Chickenpox Vaccines market report include:
- Bavarian Nordic
- Bharat Biotech
- Bio-Med Pvt Ltd
- Curevo Vaccine
- GC Biopharma (Green Cross Holdings)
- GlaxoSmithKline plc (GSK)
- Hualan Biological Engineering Inc.
- Indian Immunologicals Ltd.
- Jiangsu Yanshen Biological Technology
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma Corporation
- Novo Medi Sciences Pvt Ltd
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
- Shanghai Institute of Biological Products
- Sinovac Biotech Ltd.
- Takeda Pharmaceutical Company Ltd.
- VBI Vaccines Inc.
- Zydus Cadila
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Bavarian Nordic
- Bharat Biotech
- Bio-Med Pvt Ltd
- Curevo Vaccine
- GC Biopharma (Green Cross Holdings)
- GlaxoSmithKline plc (GSK)
- Hualan Biological Engineering Inc.
- Indian Immunologicals Ltd.
- Jiangsu Yanshen Biological Technology
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma Corporation
- Novo Medi Sciences Pvt Ltd
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
- Shanghai Institute of Biological Products
- Sinovac Biotech Ltd.
- Takeda Pharmaceutical Company Ltd.
- VBI Vaccines Inc.
- Zydus Cadila
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 374 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
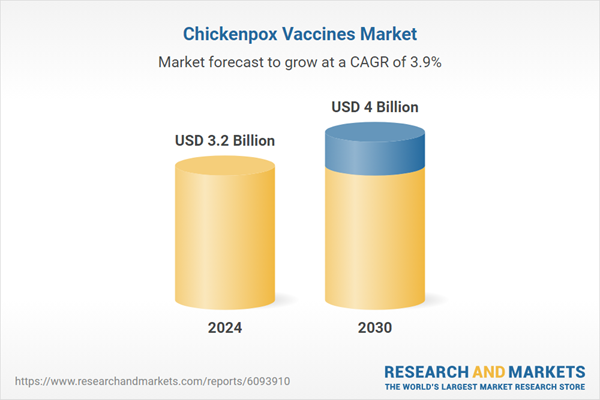
| Estimated Market Value ( USD | $ 3.2 Billion |
| Forecasted Market Value ( USD | $ 4 Billion |
| Compound Annual Growth Rate | 3.9% |
| Regions Covered | Global |