Global Ebola Drugs and Vaccines Market - Key Trends & Drivers Summarized
Why Are Ebola Drugs and Vaccines Vital in Preventing Future Outbreaks and Global Health Crises?
Ebola drugs and vaccines are central to the global response strategy against one of the world's most dangerous and highly fatal viral diseases. The Ebola virus, known for causing hemorrhagic fever and having mortality rates that can exceed 50%, poses a serious threat to public health, especially in regions with fragile healthcare infrastructure. While outbreaks have historically been confined to Central and West Africa, the interconnectedness of global travel and trade elevates the risk of cross-border transmission, making the development and deployment of effective therapeutics and vaccines a matter of international urgency. The unprecedented 2014-2016 West African outbreak, which claimed over 11,000 lives, underscored the dire need for robust medical countermeasures and catalyzed major global efforts to accelerate research, development, and approval of Ebola-specific drugs and vaccines. Treatments like monoclonal antibodies (e.g., Inmazeb and Ebanga) and vaccines such as Ervebo have since emerged as frontline tools in managing outbreaks, drastically improving survival rates and enabling more effective containment. These tools are not only life-saving but also instrumental in reducing transmission, protecting frontline health workers, and restoring public confidence during epidemic emergencies. With Ebola now considered a recurring zoonotic threat, sustained investment in drugs and vaccines is essential to prevent future health crises and maintain epidemic preparedness.How Are Scientific Advancements and Emergency Response Frameworks Driving Innovation in Ebola Therapeutics?
The Ebola drug and vaccine landscape has rapidly evolved, propelled by cutting-edge scientific innovation, global collaboration, and public-private partnerships forged in response to outbreak emergencies. Initially hindered by the sporadic and geographically limited nature of Ebola outbreaks, R&D efforts gained unprecedented momentum following the West African epidemic, leading to accelerated clinical trials and emergency use authorizations. Monoclonal antibody treatments such as Inmazeb (a cocktail of three antibodies) and Ebanga (a single antibody) have demonstrated significant efficacy in neutralizing the virus and improving survival in clinical settings. On the vaccine front, the recombinant vesicular stomatitis virus-based Ervebo vaccine has been a game-changer, providing single-dose protection and being successfully deployed in ring vaccination strategies during outbreaks in the Democratic Republic of Congo. Novel vaccine candidates using adenovirus vectors and mRNA platforms are also under development, drawing on lessons from COVID-19 vaccine development to enable faster production and broader protection. Additionally, real-time genomic surveillance and mobile diagnostic platforms are enabling more targeted therapeutic approaches and quicker outbreak identification. Organizations such as the WHO, CEPI, and GAVI play pivotal roles in coordinating global response frameworks, facilitating vaccine stockpiling, and funding accelerated development efforts. These advancements underscore the importance of sustained scientific innovation, flexible regulatory mechanisms, and coordinated response strategies in the fight against Ebola.Why Do Geographic, Economic, and Infrastructural Factors Influence Ebola Drug and Vaccine Deployment?
The deployment of Ebola drugs and vaccines is profoundly shaped by regional health infrastructure, economic capacity, and logistical readiness, with sub-Saharan Africa remaining the epicenter of disease burden and intervention efforts. Countries such as the Democratic Republic of Congo, Guinea, and Uganda have experienced multiple outbreaks and rely heavily on international aid and health partnerships for emergency response. In these areas, fragile health systems, limited cold chain capabilities, remote populations, and political instability often complicate rapid delivery and administration of life-saving interventions. Despite the availability of approved vaccines and treatments, challenges such as misinformation, vaccine hesitancy, cultural resistance, and inadequate surveillance can hinder uptake and effectiveness. In contrast, high-income countries possess the technological capacity and health infrastructure to deploy countermeasures rapidly if needed, but often deprioritize Ebola-specific resources due to lower risk profiles. International cooperation is therefore essential to bridge these disparities through initiatives such as COVAX for vaccine equity and regional manufacturing partnerships aimed at increasing local production and distribution. Moreover, training local healthcare workers, strengthening disease monitoring networks, and investing in community engagement are vital components of effective deployment. These regional nuances demonstrate that medical innovation alone is insufficient logistical coordination, socio-political strategy, and health system strengthening are equally critical in ensuring successful delivery and utilization of Ebola drugs and vaccines.What Are the Key Drivers Fueling Growth in the Ebola Drugs and Vaccines Market?
The growth in the Ebola drugs and vaccines market is driven by a combination of recurring outbreaks, heightened global health security priorities, and strategic funding from governments and international health bodies. The persistence of Ebola virus reservoirs in wildlife and the increasing frequency of spillover events from animals to humans highlight the need for continuous vigilance and preparedness. This urgency has translated into sustained investment in R&D from organizations such as the U.S. Biomedical Advanced Research and Development Authority (BARDA), the Coalition for Epidemic Preparedness Innovations (CEPI), and the World Health Organization (WHO), all of which are actively funding the development, procurement, and distribution of Ebola therapeutics and vaccines. The market is further propelled by advancements in biologics manufacturing, enabling scalable production of antibody therapies and vector-based vaccines. The COVID-19 pandemic has also increased global awareness about the necessity of robust vaccine platforms and pandemic readiness, indirectly benefiting the Ebola countermeasures pipeline by showcasing the success of rapid development and mass immunization strategies. Additionally, strategic stockpiling programs and international aid packages ensure a baseline demand for Ebola interventions, particularly in at-risk regions. Pharmaceutical companies, recognizing the potential for recurring demand, are expanding capabilities for rapid response manufacturing and regulatory agility. Together, these factors are driving a more resilient, responsive, and commercially viable market for Ebola drugs and vaccines one that is increasingly aligned with the broader goal of global health security.Key Insights:
- Market Growth: Understand the significant growth trajectory of the ZMapp segment, which is expected to reach US$47.0 Million by 2030 with a CAGR of a 23.6%. The Favipiravir segment is also set to grow at 25.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $9.9 Million in 2024, and China, forecasted to grow at an impressive 34.0% CAGR to reach $35.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Ebola Drugs and Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Ebola Drugs and Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Ebola Drugs and Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AIM ImmunoTech, Arbutus Biopharma Corporation, Bavarian Nordic A/S, BioCryst Pharmaceuticals, Inc., and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Ebola Drugs and Vaccines market report include:
- AIM ImmunoTech
- Arbutus Biopharma Corporation
- Bavarian Nordic A/S
- BioCryst Pharmaceuticals, Inc.
- Chimerix, Inc.
- Emergent BioSolutions Inc.
- Fujifilm Toyama Chemical Co., Ltd.
- GeneOne Life Sciences, Inc.
- GeoVax Labs, Inc.
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- IMV Inc.
- Johnson & Johnson
- Mapp Biopharmaceutical, Inc.
- NanoViricides, Inc.
- NewLink Genetics Corporation
- Novavax, Inc.
- Peptineo LLC
- Regeneron Pharmaceuticals, Inc.
- Sarepta Therapeutics, Inc.
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AIM ImmunoTech
- Arbutus Biopharma Corporation
- Bavarian Nordic A/S
- BioCryst Pharmaceuticals, Inc.
- Chimerix, Inc.
- Emergent BioSolutions Inc.
- Fujifilm Toyama Chemical Co., Ltd.
- GeneOne Life Sciences, Inc.
- GeoVax Labs, Inc.
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- IMV Inc.
- Johnson & Johnson
- Mapp Biopharmaceutical, Inc.
- NanoViricides, Inc.
- NewLink Genetics Corporation
- Novavax, Inc.
- Peptineo LLC
- Regeneron Pharmaceuticals, Inc.
- Sarepta Therapeutics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 485 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
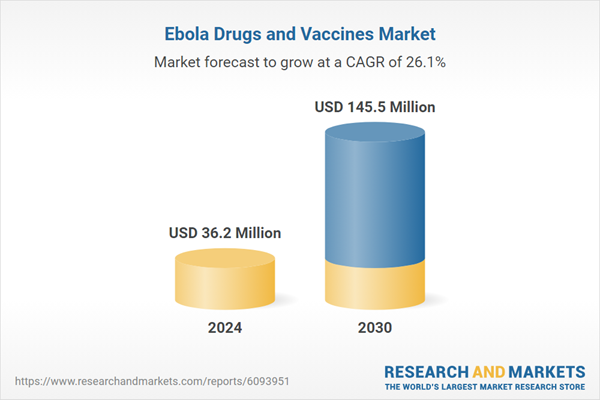
| Estimated Market Value ( USD | $ 36.2 Million |
| Forecasted Market Value ( USD | $ 145.5 Million |
| Compound Annual Growth Rate | 26.1% |
| Regions Covered | Global |