Global Stargardt Disease Therapeutics Market - Key Trends & Drivers Summarized
Why Is Stargardt Disease Drawing Increased Research and Therapeutic Development Attention?
Stargardt disease is the most common form of inherited macular degeneration, primarily affecting children and young adults. Caused by mutations in the ABCA4 gene, it leads to progressive loss of central vision due to lipofuscin accumulation in retinal pigment epithelium (RPE) cells. This accumulation damages photoreceptors, ultimately resulting in macular atrophy and legal blindness. Despite its prevalence among inherited retinal disorders, no approved treatment currently exists, making it a significant unmet medical need.Growing understanding of the molecular and genetic basis of Stargardt disease is stimulating drug development, gene therapy, and regenerative medicine efforts. Advances in imaging diagnostics - such as autofluorescence and optical coherence tomography (OCT) - are facilitating early detection and disease monitoring, essential for assessing therapeutic efficacy. As patient registries expand and regulatory incentives for orphan diseases intensify, Stargardt disease is becoming a focal point for companies developing targeted ophthalmic therapeutics.
What Novel Approaches Are Shaping the Future of Stargardt Disease Management?
Several investigational therapies are under development, including gene replacement therapies aimed at restoring functional ABCA4 expression. AAV (adeno-associated virus) vectors are commonly used for subretinal gene delivery, though large gene size remains a key challenge - prompting interest in dual-vector or non-viral delivery approaches. Emixustat, a visual cycle modulator, and other small molecule inhibitors that reduce lipofuscin formation are also progressing through clinical trials.Stem cell therapies are gaining traction as a regenerative approach, wherein RPE cells derived from embryonic or induced pluripotent stem cells (iPSCs) are transplanted into the retina to preserve vision or replace damaged cells. Additionally, CRISPR-based gene editing and antisense oligonucleotides (ASOs) are being explored to correct ABCA4 mutations or modify disease pathways. These next-generation platforms are driving optimism for long-term disease modification, especially in early-stage patients.
Where Is Therapeutic Development Advancing Across Clinical and Geographic Landscapes?
Therapeutic development is being driven by a mix of academic, biotech, and pharmaceutical collaborations, with leading institutions in North America and Europe conducting pivotal studies. Clinical trials are being coordinated through global networks of retinal specialists, genetic counselors, and patient advocacy groups to recruit diverse patient populations. The FDA and EMA have granted orphan drug designations to multiple pipeline candidates, enabling fast-track approvals and extended market exclusivity.In terms of geography, North America represents the most advanced market for Stargardt clinical research, supported by strong institutional frameworks and patient registries. Europe is active in both academic-led gene therapy studies and industrial partnerships. Asia-Pacific is beginning to see emerging interest, particularly in Japan and South Korea, where ophthalmic innovation and rare disease funding are increasing. As genetic screening becomes more accessible, broader patient identification and trial enrollment are expected globally.
What's Driving the Global Growth of the Stargardt Disease Therapeutics Market?
The growth in the global Stargardt disease therapeutics market is driven by increasing genetic diagnosis rates, rising awareness of inherited retinal diseases, and the accelerated evolution of gene-based treatments. The rarity of the condition is counterbalanced by high clinical need and long-term societal impact, positioning Stargardt disease as a strategic focus within ophthalmic drug development pipelines.Public and private funding, regulatory incentives, and growing patient advocacy are collectively accelerating innovation. As the therapeutic landscape shifts toward precision medicine and genomic intervention, Stargardt disease represents a promising model for translational research in retinal degeneration. With multiple Phase II and III trials underway and novel delivery technologies in development, the global market for Stargardt disease therapeutics is poised for substantial progress in the coming decade.
Report Scope
The report analyzes the Stargardt Disease Therapeutics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Drug Type (Emixustat, LBS-008, Other Drug Types); Age Group (Below 17 Years, Above 17 Years); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Emixustat segment, which is expected to reach US$1.2 Billion by 2030 with a CAGR of a 33.9%. The LBS-008 segment is also set to grow at 29.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $98.1 Million in 2024, and China, forecasted to grow at an impressive 41.5% CAGR to reach $486 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Stargardt Disease Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Stargardt Disease Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Stargardt Disease Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Advanced Circuits, ALLPCB, American Standard Circuits, Amphenol Printed Circuits, AT&S Austria Technologie & Systemtechnik AG and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Stargardt Disease Therapeutics market report include:
- 4P-Pharma
- Alkeus Pharmaceuticals
- Ascidian Therapeutics
- Astellas Pharma
- Belite Bio
- Biophytis
- Clearside Biomedical
- Gene Vector Technologies
- IVERIC bio
- Kiora Pharmaceuticals
- Kubota Vision
- MD Stem Cells
- Mediphage Bioceuticals
- Nanoscope Therapeutics
- Novartis
- Ocugen
- Oxford Biomedica
- reVision Therapeutics
- SalioGen Therapeutics
- SpliceBio
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 4P-Pharma
- Alkeus Pharmaceuticals
- Ascidian Therapeutics
- Astellas Pharma
- Belite Bio
- Biophytis
- Clearside Biomedical
- Gene Vector Technologies
- IVERIC bio
- Kiora Pharmaceuticals
- Kubota Vision
- MD Stem Cells
- Mediphage Bioceuticals
- Nanoscope Therapeutics
- Novartis
- Ocugen
- Oxford Biomedica
- reVision Therapeutics
- SalioGen Therapeutics
- SpliceBio
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 364 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
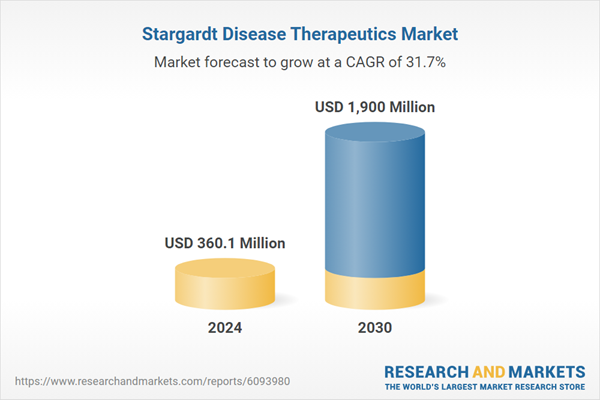
| Estimated Market Value ( USD | $ 360.1 Million |
| Forecasted Market Value ( USD | $ 1900 Million |
| Compound Annual Growth Rate | 31.7% |
| Regions Covered | Global |