Global 'Sodium Phenylbutyrate' Market - Key Trends & Drivers Summarized
Can Sodium Phenylbutyrate Unlock New Frontiers In Rare Disease And Metabolic Therapy?
Sodium phenylbutyrate (NaPB) is a niche yet critically important pharmaceutical compound used in the treatment of urea cycle disorders (UCDs) - a group of rare genetic conditions that hinder the body's ability to remove waste nitrogen. As a nitrogen-scavenging agent, NaPB facilitates the excretion of ammonia through alternative metabolic pathways, preventing neurotoxicity and systemic complications. Beyond UCDs, research has uncovered potential uses in spinal muscular atrophy, amyotrophic lateral sclerosis (ALS), cystic fibrosis, and various cancers - particularly due to NaPB's role as a histone deacetylase (HDAC) inhibitor. While current market volumes are relatively small compared to mainstream pharmaceuticals, NaPB's designation as an orphan drug has made it a target for biotech firms and specialty pharma companies like Horizon Therapeutics and Recordati Rare Diseases. With the expansion of newborn screening and rare disease awareness, sodium phenylbutyrate is evolving from a niche therapy into a broader metabolic modulator with high clinical significance.How Are Orphan Drug Incentives Accelerating Market Expansion?
Global regulatory frameworks around orphan drugs - particularly in the U.S. and EU - are encouraging research, development, and commercialization of treatments like sodium phenylbutyrate for rare conditions. These incentives include tax credits, extended market exclusivity, and fast-track approval processes. As more patients are diagnosed early via neonatal screening or genetic testing, demand for NaPB is becoming more consistent and predictable. Additionally, NaPB's reformulation into palatable, easy-to-administer versions (like granules or oral liquids) is improving pediatric compliance and accessibility. Partnerships between pharma firms and patient advocacy groups are further boosting diagnosis and access. The evolving reimbursement landscape, particularly in North America and Europe, is making high-cost orphan drugs more viable through negotiated pricing and specialty pharmacy distribution models. As healthcare systems invest more in precision medicine and personalized care, orphan drugs like NaPB are gaining stronger footholds in formularies and clinical protocols.Is Clinical Research Unlocking New Indications For Sodium Phenylbutyrate?
The pharmacological profile of sodium phenylbutyrate is expanding beyond its traditional role in metabolic disorders. Its HDAC-inhibiting properties have made it a candidate for ongoing trials in oncology, neurology, and inflammatory diseases. In combination with other compounds, NaPB has shown promise in regulating gene expression, reducing fibrosis, and modulating immune response in preclinical and Phase I/II studies. Cystic fibrosis, sickle cell anemia, and neurodegenerative conditions such as Alzheimer's and Parkinson's are being explored as potential therapeutic domains. These research initiatives are positioning sodium phenylbutyrate not only as a metabolic scavenger but as a multi-mechanism therapeutic agent. While still in early stages for many applications, successful trial outcomes could significantly widen the market scope, transitioning the compound from rare disease therapy into a broader category of epigenetic and systemic disorder treatments.The Growth In The Sodium Phenylbutyrate Market Is Driven By Several Factors - What's Expanding Its Clinical Relevance?
The growth in the sodium phenylbutyrate market is driven by several factors, including rising diagnosis rates of urea cycle disorders, improved access to genetic testing, and growing acceptance of orphan drug pricing models. Regulatory support in the form of fast-track approvals, orphan designation, and expanded reimbursement is making rare disease therapy development more feasible. In addition, increasing pharmaceutical research into epigenetic mechanisms and metabolic modulation is driving interest in NaPB's broader applications. Improved drug formulations and distribution through specialty channels are enhancing patient access and compliance. With ongoing clinical trials investigating its use in neurological, pulmonary, and oncological indications, sodium phenylbutyrate is set to evolve from a specialized treatment into a multi-indication therapeutic candidate.Report Scope
The report analyzes the Sodium Phenylbutyrate market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Application (Pharmaceutical, Food & Beverages, Other Applications); Distribution Channel (Direct Sales, Distributor Sales, Online Retail); End-User (Hospitals & Clinics, Research Institutes, Food & Beverages, Cosmetics, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 44 companies featured in this Sodium Phenylbutyrate market report include -
- Acer Therapeutics Inc.
- Alvogen, Inc.
- Amylyx Pharmaceuticals Inc.
- Apothecon Pharmaceuticals
- Darmerica LLC
- Dipharma Francis S.r.l.
- Glenmark Pharmaceuticals Ltd.
- Horizon Therapeutics plc
- HRV Global Life Sciences
- Medisca Inc.
- Medunik Canada
- Metrochem API Pvt. Ltd.
- Nuray Chemicals Pvt. Ltd.
- Par Pharmaceutical Companies, Inc.
- Patheon Inc.
- Scandinavian Formulas Inc.
- Seqens Group
- Shodhana Laboratories Pvt. Ltd.
- Sigmapharm Laboratories, LLC
- Stellence Pharmscience Pvt. Ltd.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Direct Sales Channel segment, which is expected to reach US$1.7 Billion by 2030 with a CAGR of a 6.9%. The Distributor Sales Channel segment is also set to grow at 4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $542.5 Million in 2024, and China, forecasted to grow at an impressive 9.4% CAGR to reach $573 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Sodium Phenylbutyrate Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Sodium Phenylbutyrate Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Sodium Phenylbutyrate Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as A.H.A International Co., Ltd., Aditya Birla Chemicals, Akash Purochem Private Limited, Angel Chemicals, ChemCeed and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 44 Featured):
- Acer Therapeutics Inc.
- Alvogen, Inc.
- Amylyx Pharmaceuticals Inc.
- Apothecon Pharmaceuticals
- Darmerica LLC
- Dipharma Francis S.r.l.
- Glenmark Pharmaceuticals Ltd.
- Horizon Therapeutics plc
- HRV Global Life Sciences
- Medisca Inc.
- Medunik Canada
- Metrochem API Pvt. Ltd.
- Nuray Chemicals Pvt. Ltd.
- Par Pharmaceutical Companies, Inc.
- Patheon Inc.
- Scandinavian Formulas Inc.
- Seqens Group
- Shodhana Laboratories Pvt. Ltd.
- Sigmapharm Laboratories, LLC
- Stellence Pharmscience Pvt. Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Acer Therapeutics Inc.
- Alvogen, Inc.
- Amylyx Pharmaceuticals Inc.
- Apothecon Pharmaceuticals
- Darmerica LLC
- Dipharma Francis S.r.l.
- Glenmark Pharmaceuticals Ltd.
- Horizon Therapeutics plc
- HRV Global Life Sciences
- Medisca Inc.
- Medunik Canada
- Metrochem API Pvt. Ltd.
- Nuray Chemicals Pvt. Ltd.
- Par Pharmaceutical Companies, Inc.
- Patheon Inc.
- Scandinavian Formulas Inc.
- Seqens Group
- Shodhana Laboratories Pvt. Ltd.
- Sigmapharm Laboratories, LLC
- Stellence Pharmscience Pvt. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 287 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
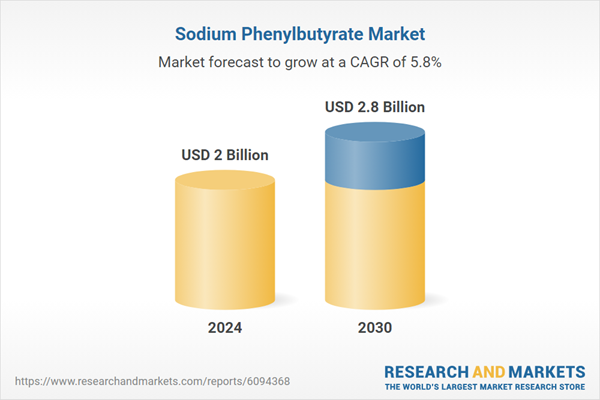
| Estimated Market Value ( USD | $ 2 Billion |
| Forecasted Market Value ( USD | $ 2.8 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |