Global Bird Flu Treatment Market - Key Trends & Drivers Summarized
Why Is Bird Flu Treatment an Urgent Focus for Global Public Health and Biopharmaceutical Innovation?
Bird flu, or avian influenza, remains a persistent and evolving threat to both global public health and the poultry industry. Caused by influenza type A viruses such as H5N1, H7N9, and others, bird flu primarily affects birds but has the capacity to infect humans through close contact with infected animals or contaminated environments. Human infections, though relatively rare, often result in severe respiratory illness and a high mortality rate, especially in the case of highly pathogenic avian influenza (HPAI) strains. With repeated outbreaks occurring in Asia, Europe, Africa, and the Americas, the demand for effective bird flu treatments and rapid response strategies has never been greater. The zoonotic nature of the virus - its ability to jump from animals to humans - poses a significant pandemic risk, as evidenced by past influenza pandemics. Moreover, the increasing prevalence of drug-resistant strains and genetic mutations in avian influenza viruses complicate treatment efforts and underline the urgency of developing novel antiviral drugs, therapeutic antibodies, and supportive care protocols. Treatment typically includes neuraminidase inhibitors like oseltamivir and zanamivir, though their effectiveness varies depending on the strain and timing of administration. In addition to pharmacologic interventions, supportive therapies such as oxygen supplementation, fluid management, and, in severe cases, mechanical ventilation, are crucial. The bird flu treatment market is not just a healthcare issue but a critical component of biosecurity and pandemic preparedness strategies worldwide.How Are Scientific and Technological Advances Transforming the Landscape of Bird Flu Therapeutics?
Scientific innovation is playing a pivotal role in reshaping the future of bird flu treatment by expanding both the effectiveness and scope of therapeutic options. Traditional antiviral drugs such as oseltamivir (Tamiflu) and zanamivir (Relenza) continue to be used as first-line treatments, but growing resistance among certain H5 and H7 strains has prompted the development of new drug classes and combination therapies. Researchers are now exploring polymerase inhibitors, monoclonal antibodies, and host-directed therapies that target cellular pathways involved in viral replication. mRNA-based therapeutics, inspired by the success of COVID-19 vaccines, are being adapted to rapidly target emerging avian influenza strains with customized protein antigens or immune-modulating components. In addition to antivirals, convalescent plasma therapy and immunoglobulin treatments are being investigated for their ability to boost patient recovery during severe infections. Diagnostic advancements, particularly in molecular diagnostics like RT-PCR and CRISPR-based point-of-care tools, allow for early detection of infection and timely initiation of treatment - an essential factor in reducing complications. AI and bioinformatics are being utilized to map viral evolution and predict mutation patterns, supporting the design of future-proof treatments and guiding global stockpiling strategies. Additionally, collaborations between global health organizations and pharmaceutical companies are accelerating clinical trials and the approval process for bird flu therapeutics. These innovations are not only enhancing clinical outcomes but also preparing healthcare systems for a more agile and effective response to future avian influenza outbreaks.Which Global Markets and Health Systems Are Leading the Effort in Bird Flu Treatment and Preparedness?
Efforts to advance bird flu treatment and outbreak response are concentrated in regions with high poultry density, previous outbreak history, and strong healthcare infrastructure. Countries in East and Southeast Asia - including China, Vietnam, Indonesia, and Thailand - are at the forefront of surveillance and treatment development due to their frequent exposure to bird flu outbreaks in both wild and domesticated bird populations. China, in particular, has invested heavily in avian influenza research, diagnostics, and vaccine production, especially following large-scale outbreaks of H7N9. Japan and South Korea are also notable for their integrated strategies combining early detection, antiviral stockpiling, and public health coordination. In North America, the United States and Canada maintain robust preparedness frameworks under the guidance of agencies like the CDC and USDA, focusing on rapid response, antiviral distribution, and strategic national stockpiles. The U.S. Biomedical Advanced Research and Development Authority (BARDA) supports bird flu treatment R&D through public-private partnerships and federal funding. Europe, led by countries such as the UK, France, and Germany, emphasizes One Health approaches, integrating human, animal, and environmental health surveillance to manage zoonotic threats. Meanwhile, international organizations like the WHO, FAO, and OIE (now WOAH) play a central role in coordinating global responses, providing technical guidance, and supporting treatment accessibility in developing nations. Sub-Saharan Africa and parts of South America are emerging areas of concern, where limited healthcare infrastructure poses challenges but also presents opportunities for targeted intervention and capacity-building. Across all these regions, the integration of treatment protocols with real-time outbreak intelligence and policy frameworks is essential to mitigating the impacts of bird flu and preventing its escalation into a broader health crisis.What Is Fueling the Growth in the Global Bird Flu Treatment Market?
The growth in the global bird flu treatment market is driven by a combination of epidemiological urgency, scientific advancement, policy focus, and increased investment in pandemic preparedness. The repeated emergence of highly pathogenic avian influenza outbreaks in both developed and developing countries has heightened global awareness of the need for rapid, effective therapeutic options. The potential for bird flu strains to mutate and cause a human pandemic - especially as they acquire capabilities for sustained human-to-human transmission - has made treatment development a top priority for public health agencies and pharmaceutical companies alike. Government funding and strategic health initiatives are also playing a critical role, with national stockpiling programs, emergency use authorizations, and grants for antiviral R&D providing a solid foundation for market growth. The success of mRNA and monoclonal antibody platforms in combating other viral infections has accelerated interest and investment in similar technologies for avian influenza. Additionally, the growing demand for personalized and precision medicine is influencing the design of targeted therapeutics that account for individual immune responses and viral subtypes. The rise of global travel, international poultry trade, and urbanization are increasing the risk of virus transmission, further driving the need for robust treatment infrastructure. Furthermore, advancements in data analytics, epidemiological modeling, and digital health tools are supporting faster identification of outbreaks and streamlined therapeutic deployment. Collectively, these factors are not only expanding the market for bird flu treatments but are also embedding it into broader public health strategies aimed at building global resilience against infectious disease threats.Report Scope
The report analyzes the Bird Flu Treatment market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Antiviral Treatment, Combination Treatment, Prophylactic Antibiotics Treatment, Ribavirin Treatment); End-Use (Clinics End-Use, Hospitals End-Use, Institutional Health Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Antiviral Treatment segment, which is expected to reach US$13.8 Billion by 2030 with a CAGR of a 5%. The Combination Treatment segment is also set to grow at 4.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $6.8 Billion in 2024, and China, forecasted to grow at an impressive 8.8% CAGR to reach $6.9 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bird Flu Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bird Flu Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bird Flu Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Ashland Global Holdings Inc., BASF SE, Bezwada Biomedical LLC, Corbion N.V. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Bird Flu Treatment market report include:
- Abbott
- BioCryst Pharmaceuticals
- Boehringer Ingelheim
- Ceva Santé Animale
- Cipla
- CSL Limited (Seqirus)
- Elanco Animal Health
- Gilead Sciences
- GlaxoSmithKline (GSK)
- Hester Biosciences
- Macleods Pharmaceuticals
- Merck & Co.
- Moderna
- Novavax
- Pfizer
- Phibro Animal Health
- Roche
- Sanofi
- Sinovac Biotech
- Zoetis
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott
- BioCryst Pharmaceuticals
- Boehringer Ingelheim
- Ceva Santé Animale
- Cipla
- CSL Limited (Seqirus)
- Elanco Animal Health
- Gilead Sciences
- GlaxoSmithKline (GSK)
- Hester Biosciences
- Macleods Pharmaceuticals
- Merck & Co.
- Moderna
- Novavax
- Pfizer
- Phibro Animal Health
- Roche
- Sanofi
- Sinovac Biotech
- Zoetis
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 285 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
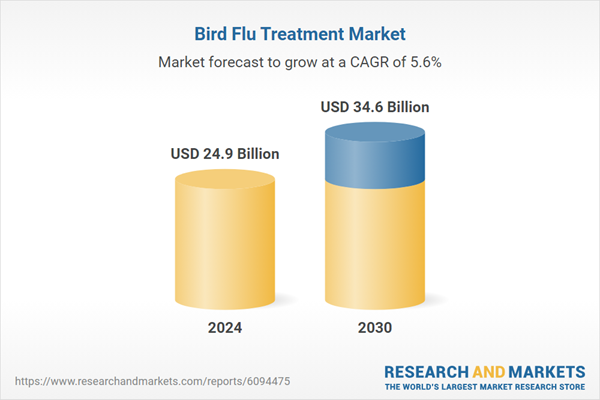
| Estimated Market Value ( USD | $ 24.9 Billion |
| Forecasted Market Value ( USD | $ 34.6 Billion |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |