Global Bladder Volume Manometer Catheters Market - Key Trends & Drivers Summarized
Why Are Bladder Volume Manometer Catheters Gaining Prominence in Urological Diagnostics?
Bladder volume manometer catheters have emerged as indispensable tools in modern urology, primarily due to their dual functionality of measuring bladder pressure and volume with high precision. These specialized catheters are playing an increasingly vital role in diagnosing lower urinary tract dysfunctions such as neurogenic bladder, urinary incontinence, bladder outlet obstruction, and detrusor overactivity. Their prominence is rising in both inpatient and outpatient clinical settings, particularly in urodynamic studies, where accurate data is critical for forming treatment strategies. Unlike conventional urinary catheters that merely drain urine, these devices provide real-time pressure readings while simultaneously monitoring bladder capacity, offering clinicians a dynamic picture of bladder function under different physiological conditions. This added diagnostic value is crucial for tailoring individualized treatment regimens and surgical planning, particularly for patients with complex conditions such as spinal cord injuries, Parkinson's disease, or post-stroke urological disorders. With the rise in aging populations globally - who are more susceptible to such disorders - the demand for precise, minimally invasive diagnostics has grown significantly. Moreover, the integration of digital pressure transducers and electronic data capture in newer catheter systems is streamlining the diagnostic workflow, enhancing clinical decision-making, and reducing patient discomfort. As urological practices shift towards more evidence-based care models, bladder volume manometer catheters are becoming essential in standardizing and improving the quality of diagnostic protocols.How Are Technological Advancements Transforming Catheter Precision and Patient Safety?
Recent technological advancements in bladder volume manometer catheter systems are redefining the standards for accuracy, usability, and patient safety. Innovations in sensor miniaturization, material science, and catheter ergonomics have significantly improved device performance and patient tolerance. High-fidelity pressure transducers embedded in catheter tips now deliver more precise pressure readings with minimal signal noise, enhancing diagnostic reliability. In parallel, manufacturers are developing dual-lumen and triple-lumen catheters that allow for simultaneous bladder filling, pressure monitoring, and optional therapeutic intervention, thereby reducing procedural time and the need for multiple insertions. Biocompatible and hypoallergenic materials are being adopted to reduce the risk of catheter-associated urinary tract infections (CAUTIs), a major concern in both acute and chronic care settings. Many catheters now come with anti-microbial coatings or silver ion technology, which further enhances infection control without compromising catheter functionality. Additionally, the integration of Bluetooth-enabled pressure monitoring and wireless data transmission is paving the way for remote diagnostics and tele-urodynamics, allowing urologists to monitor patients in real time from decentralized locations. These technological strides are not only improving clinical outcomes but also increasing patient satisfaction by minimizing invasiveness and procedural discomfort. With hospitals and diagnostic centers under pressure to enhance efficiency and reduce hospital-acquired complications, bladder volume manometer catheters equipped with cutting-edge features are fast becoming the preferred choice for modern urodynamic assessments.What Role Do Clinical Applications and End-User Demand Play in Market Expansion?
The clinical utility of bladder volume manometer catheters extends across a wide spectrum of diagnostic and therapeutic scenarios, and this versatility is fueling significant market expansion. Hospitals, diagnostic centers, long-term care facilities, and rehabilitation clinics are the primary end-users of these catheters, employing them in urodynamic testing, post-operative monitoring, and chronic bladder dysfunction management. As awareness of the importance of early and accurate diagnosis of urinary disorders grows among healthcare providers, so too does the integration of advanced catheter systems into routine care pathways. Pediatric urology and geriatrics are two particularly fast-growing segments, where bladder dysfunctions often go underdiagnosed but have substantial impact on quality of life and healthcare costs. Moreover, the increasing prevalence of neurological diseases such as multiple sclerosis, spinal cord injury, and diabetic neuropathy is driving consistent demand for bladder monitoring tools that can guide both pharmacological and surgical interventions. Health systems are recognizing the value of early urodynamic screening in preventing complications such as upper urinary tract damage and renal impairment, which can result from unmanaged bladder dysfunction. Training programs and continuing medical education (CME) modules are also promoting the adoption of bladder volume manometer catheters by improving clinician familiarity and procedural confidence. With rising healthcare expenditure in both developed and emerging economies, and the growing emphasis on personalized and preventive medicine, demand for these high-utility diagnostic devices continues to rise, supported by an increasingly diversified end-user base.What Are the Key Drivers Behind the Rising Demand for Bladder Volume Manometer Catheters?
The growth in the bladder volume manometer catheters market is driven by several factors related to technological evolution, demographic shifts, healthcare policy changes, and clinical awareness. One of the primary drivers is the global rise in urinary disorders linked to aging populations, sedentary lifestyles, and chronic diseases such as diabetes and neurological conditions, all of which increase the need for precision urodynamic evaluations. On the technological front, significant improvements in catheter design - such as enhanced flexibility, higher resolution sensors, and digital interfacing - are making these devices more effective and user-friendly, encouraging wider clinical adoption. Hospitals and diagnostic labs are also investing in sophisticated urodynamic systems that rely on these catheters as integral components, further embedding them into standard diagnostic workflows. Regulatory bodies in North America, Europe, and parts of Asia are issuing updated clinical guidelines that emphasize the importance of bladder pressure and volume monitoring in both acute and chronic care settings, indirectly bolstering market growth. Insurance coverage and reimbursement pathways for urodynamic procedures have also improved in several countries, making these diagnostics more financially accessible for both institutions and patients. Simultaneously, the emergence of portable and home-based urodynamic kits, enabled by wireless catheter systems, is creating new application environments, especially for remote care and home monitoring. Strategic collaborations between medtech companies and healthcare institutions are accelerating innovation and market reach. Collectively, these drivers - ranging from clinical necessity and patient safety to regulatory support and technological innovation - are catalyzing robust and sustained growth in the global bladder volume manometer catheters market.Report Scope
The report analyzes the Bladder Volume Manometer Catheters market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Indwelling Catheters, Intermittent Catheters, External Catheters); Application (Urinary Incontinence Application, Benign Prostatic Hyperplasia Application, Surgery Application, Other Applications); End-Use (Hospitals End-Use, Ambulatory Surgery Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Indwelling Catheters segment, which is expected to reach US$409.3 Million by 2030 with a CAGR of a 3.9%. The Intermittent Catheters segment is also set to grow at 4.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $154.7 Million in 2024, and China, forecasted to grow at an impressive 7.5% CAGR to reach $147.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bladder Volume Manometer Catheters Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bladder Volume Manometer Catheters Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bladder Volume Manometer Catheters Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as A&A Fratelli Parodi Spa, AAK AB, All Organic Treasures GmbH, Aromaaz International, Bioriginal Food & Science Corp and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Bladder Volume Manometer Catheters market report include:
- Ameco Medical Industries
- Asid Bonz GmbH
- B. Braun Melsungen AG
- Bactiguard AB
- Boston Scientific Corporation
- Cardinal Health
- Coloplast A/S
- Cook Medical
- Hamilton Company
- Hi-Tech Assembly Systems Inc.
- Hollister Incorporated
- Lilium Otsuka Co., Ltd.
- Medax International Inc.
- Medica Group PLC
- Mednova Ltd.
- Medtronic plc
- Ribbel International Limited
- Teleflex Incorporated
- Urocare Products, Inc.
- UroMed Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Ameco Medical Industries
- Asid Bonz GmbH
- B. Braun Melsungen AG
- Bactiguard AB
- Boston Scientific Corporation
- Cardinal Health
- Coloplast A/S
- Cook Medical
- Hamilton Company
- Hi-Tech Assembly Systems Inc.
- Hollister Incorporated
- Lilium Otsuka Co., Ltd.
- Medax International Inc.
- Medica Group PLC
- Mednova Ltd.
- Medtronic plc
- Ribbel International Limited
- Teleflex Incorporated
- Urocare Products, Inc.
- UroMed Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 383 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
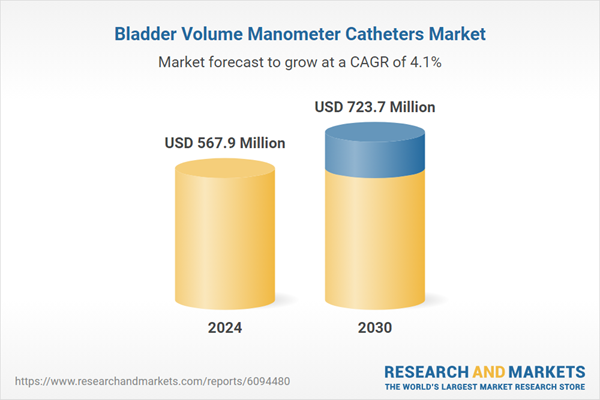
| Estimated Market Value ( USD | $ 567.9 Million |
| Forecasted Market Value ( USD | $ 723.7 Million |
| Compound Annual Growth Rate | 4.1% |
| Regions Covered | Global |