Global Bowie Dick Test Packs Market - Key Trends & Drivers Summarized
What Makes Bowie Dick Test Packs Indispensable in Modern Sterilization Protocols?
Bowie Dick test packs play a pivotal role in ensuring the reliability and safety of hospital sterilization processes, particularly in steam sterilizers used for surgical instruments and other medical equipment. These test packs are designed to detect air leaks and ineffective air removal from the sterilizer chamber, a crucial requirement for achieving proper steam penetration and uniform sterilization. Without complete air removal, sterilization efficacy can be severely compromised, placing patient safety at risk. The Bowie Dick test, named after its creators Dr. J. Bowie and J. Dick, has become a standard diagnostic tool, especially for pre-vacuum steam sterilizers. It is a mandatory component of daily checks in most healthcare facilities, and its usage is mandated by organizations like AAMI, CDC, and WHO in sterilization quality assurance protocols. While early versions were handmade with towels and indicator paper, today's commercial Bowie Dick test packs are preassembled, standardized, and compliant with ISO 11140-4, ensuring consistent performance across applications. These packs simulate the most challenging conditions for steam penetration, acting as an early warning system for system failures. They help identify issues such as residual air pockets, non-condensable gases, or vacuum pump malfunctions before actual instrument sterilization begins. Their growing usage is not limited to large hospitals; outpatient surgery centers, dental clinics, and ambulatory care units increasingly rely on these packs to meet stringent infection control standards. The importance of maintaining a zero-tolerance approach to sterilization failures has propelled the test pack from a regulatory obligation to a daily operational safeguard. In a healthcare landscape increasingly focused on accountability, traceability, and performance validation, Bowie Dick test packs represent the frontline defense against sterilization lapses.How Are Regulatory Standards Shaping the Adoption of Bowie Dick Testing?
Stringent regulatory requirements and international sterilization standards have been a dominant force behind the growing adoption and standardization of Bowie Dick test packs across healthcare institutions. Guidelines issued by organizations such as the International Organization for Standardization (ISO), the Association for the Advancement of Medical Instrumentation (AAMI), and local health ministries mandate daily vacuum leak tests to validate the efficiency of pre-vacuum steam sterilizers. These guidelines recommend or require that Bowie Dick tests be performed at the beginning of each sterilization day before processing actual medical loads. As global regulatory environments tighten in response to concerns over hospital-acquired infections (HAIs) and surgical site infections (SSIs), compliance with sterilization testing has become non-negotiable. In particular, ISO 17665 and ISO 11140 standards have brought clarity and uniformity to sterilization quality control, ensuring that Bowie Dick packs function as reliable diagnostic tools rather than procedural formalities. Moreover, healthcare accreditation bodies such as the Joint Commission (USA), NABH (India), and CHKS (UK) increasingly audit sterilization validation records, making accurate and timely Bowie Dick testing a benchmark for accreditation readiness. These standards have also prompted many healthcare facilities to transition from improvised or homemade test packs to commercially manufactured ones that provide validated, reproducible results. In turn, manufacturers have responded with test packs that deliver quick visual indicators, user-friendly interfaces, and compatibility with record-keeping systems. Furthermore, training and certification programs for Central Sterile Services Department (CSSD) personnel now emphasize the correct usage and interpretation of Bowie Dick test results as part of professional competency. This regulatory landscape has made test packs not just quality tools, but compliance essentials, deeply embedding them in the infrastructure of modern sterilization protocols.Are Technological Innovations Enhancing the Performance and Reliability of Test Packs?
The evolution of Bowie Dick test packs has kept pace with broader technological advancements in sterilization science, resulting in test products that are more accurate, efficient, and traceable than ever before. Modern test packs now incorporate advanced chemical indicators with precise color-change thresholds, enabling clearer and more consistent interpretation of results. Unlike older test sheets that required subjective judgment, today's packs often include printed color reference charts or digital interpretation aids to eliminate ambiguity and reduce human error. Some of the latest developments include electronic Bowie Dick test systems, which use sensors and digital monitoring to log sterilization data, detect pressure fluctuations, and record test outcomes automatically. These innovations are particularly valuable in large hospitals and surgical centers where automation and traceability are paramount. Test packs are also being designed to work seamlessly with sterilizers equipped with barcode scanning and software integration, allowing sterilization teams to track and archive test results electronically for audits and compliance documentation. Additionally, improvements in packaging materials and construction have led to test packs that are more environmentally friendly, have a longer shelf life, and offer greater resistance to humidity and physical damage. Manufacturers are also focusing on creating compact, low-waste packs that reduce storage needs and disposal costs without compromising diagnostic accuracy. With increasing demand for sustainability and digital compatibility in healthcare products, such innovations position modern Bowie Dick test packs as both high-performance tools and integral components of sterilization workflow automation. These advancements help healthcare institutions not only comply with safety protocols but also improve operational efficiency and infection control outcomes.What Factors Are Driving Market Growth and Demand Across Healthcare Systems?
The growth in the Bowie Dick test packs market is driven by several factors directly tied to healthcare infrastructure expansion, infection control priorities, and technology integration in sterilization workflows. First, the rising number of surgical procedures and the growing complexity of reusable medical instruments have amplified the demand for reliable sterilization validation tools. With hospital-acquired infections posing a major challenge to patient safety, healthcare systems worldwide are investing more heavily in quality assurance practices, and Bowie Dick test packs are central to those efforts. Second, the global push for improved infection control, especially in the wake of COVID-19, has intensified scrutiny on sterilization protocols, leading to more rigorous testing regimens. As a result, even smaller clinics, dental practices, and outpatient centers are adopting Bowie Dick packs as part of their daily routine. Third, the proliferation of centralized sterile services departments (CSSDs) and automated sterilization cycles has created a need for diagnostic tools that integrate easily into standardized workflows. Additionally, the availability of a wide range of test pack formats - from single-use to reprocessable variants - has made them accessible across various budget levels and clinical settings. Government-backed healthcare modernization programs in emerging economies are further stimulating market penetration, particularly where infrastructure upgrades include investment in new sterilization equipment. Moreover, the increasing use of third-party sterilization and decontamination services by hospitals and surgical centers necessitates validated test protocols, fueling consistent demand for test packs. Finally, awareness initiatives and training programs led by infection control associations and sterilization equipment manufacturers are promoting best practices, thereby reinforcing the role of Bowie Dick test packs as essential safety tools. This convergence of clinical need, regulatory oversight, and technological advancement is ensuring a steady upward trajectory for the global Bowie Dick test packs market.Report Scope
The report analyzes the Bowie Dick Test Packs market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Type 1 Test Packs, Type 2 Test Packs, Type 3 Test Packs, Type 4 Test Packs); End-Use (Pharma & Medical Device Companies End-Use, Healthcare Facilities End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Type 1 Test Packs segment, which is expected to reach US$148.1 Million by 2030 with a CAGR of a 5.9%. The Type 2 Test Packs segment is also set to grow at 6.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $58 Million in 2024, and China, forecasted to grow at an impressive 8.9% CAGR to reach $59.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bowie Dick Test Packs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bowie Dick Test Packs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bowie Dick Test Packs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABC Medical, Inc., Aquaflush Medical Limited, Axonics, Inc., B. Braun Melsungen AG, Becton, Dickinson and Company and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Bowie Dick Test Packs market report include:
- 3M
- BEYA Medical
- Crosstex International, Inc.
- EDM3 Solutions
- EFELAB SRL
- Famos Medizintechnik Vertriebs GmbH
- Getinge AB
- Hawo GmbH
- Mediwish Co., Ltd.
- Medline Industries, LP
- Mesa Laboratories, Inc.
- NiGK Corporation
- PMS Healthcare Technologies
- Premeds
- Propper Manufacturing Co., Inc.
- Rodwell Autoclave Company
- Sanax Inc.
- Sterdoc
- STERIS Corporation
- Terragene
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M
- BEYA Medical
- Crosstex International, Inc.
- EDM3 Solutions
- EFELAB SRL
- Famos Medizintechnik Vertriebs GmbH
- Getinge AB
- Hawo GmbH
- Mediwish Co., Ltd.
- Medline Industries, LP
- Mesa Laboratories, Inc.
- NiGK Corporation
- PMS Healthcare Technologies
- Premeds
- Propper Manufacturing Co., Inc.
- Rodwell Autoclave Company
- Sanax Inc.
- Sterdoc
- STERIS Corporation
- Terragene
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 288 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
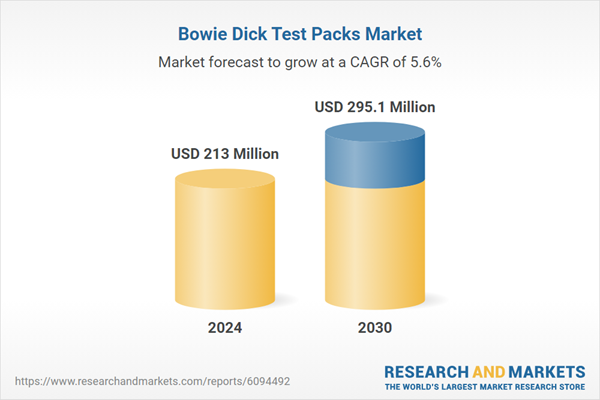
| Estimated Market Value ( USD | $ 213 Million |
| Forecasted Market Value ( USD | $ 295.1 Million |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |