Global Breast Recurrence Score Test Market - Key Trends & Drivers Summarized
How Is Genomic Profiling Revolutionizing Breast Cancer Prognosis and Treatment Planning?
The Breast Recurrence Score Test, most commonly associated with genomic assays like Oncotype DX, has dramatically transformed how clinicians assess the risk of breast cancer recurrence and make critical decisions about adjuvant therapy. This test evaluates the expression of a specific panel of cancer-related genes - typically 21 genes - to produce a personalized recurrence score that estimates the likelihood of distant cancer recurrence within ten years of initial treatment. Unlike traditional prognostic tools that rely heavily on clinical and pathological features such as tumor size, lymph node involvement, and hormone receptor status, the Breast Recurrence Score Test provides a molecular-level insight into tumor biology. This enables oncologists to better stratify patients into low, intermediate, or high-risk categories, tailoring treatment plans accordingly. For example, patients with a low recurrence score can often forgo chemotherapy, thus avoiding its considerable side effects without compromising survival outcomes. This has marked a significant paradigm shift in breast cancer care, moving toward precision oncology that emphasizes individualized treatment over one-size-fits-all protocols. The growing body of clinical evidence from landmark trials such as TAILORx and RxPONDER has validated the clinical utility of the test, boosting physician and patient confidence in its results. As awareness of the test's capabilities grows, it is increasingly being integrated into standard care protocols for early-stage, hormone receptor-positive, HER2-negative breast cancer. The result is not just improved clinical outcomes, but enhanced patient satisfaction and quality of life. The test also aligns with the shift toward value-based healthcare, by enabling more cost-effective treatment planning and reducing unnecessary therapeutic interventions.What Role Are Clinical Guidelines and Reimbursement Policies Playing in Driving Adoption?
The increasing integration of the Breast Recurrence Score Test into clinical guidelines and insurance reimbursement frameworks has played a critical role in accelerating its global adoption. Esteemed bodies such as the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and the St. Gallen International Expert Consensus have incorporated the test into their formal guidelines for managing hormone receptor-positive, HER2-negative breast cancer. These endorsements have lent strong clinical credibility to the test, encouraging oncologists across the globe to adopt it as a routine diagnostic tool. In tandem, many public and private insurers - particularly in the U.S., Canada, and parts of Europe - have approved reimbursement for the test, recognizing its long-term value in improving patient outcomes and minimizing overtreatment. Reimbursement policies have a direct influence on test utilization, especially in countries with centralized healthcare systems, where approved diagnostic tests are more likely to be widely adopted. Moreover, expanding insurance coverage has facilitated access to the test for underserved populations, making precision oncology more equitable. Policy decisions have also been guided by economic evaluations that show the test can significantly reduce the costs associated with unnecessary chemotherapy, hospital stays, and long-term management of treatment-related side effects. In emerging markets, the inclusion of such genomic assays in public health strategies is slower but steadily increasing, as local regulatory bodies assess clinical data and cost-effectiveness reports. Additionally, pharmaceutical companies and diagnostic firms are actively engaging with health ministries and payers to establish pathways for test adoption. The alignment of medical necessity, policy support, and cost-containment objectives has solidified the Breast Recurrence Score Test's place in contemporary cancer care.Are Technological Advancements Enhancing Test Accuracy and Global Accessibility?
The evolution of molecular diagnostics technology has greatly enhanced the accuracy, scalability, and accessibility of Breast Recurrence Score Tests. Originally processed only in centralized labs with long turnaround times, newer iterations benefit from streamlined sample processing, faster gene expression analysis, and increasingly robust algorithms that ensure precise recurrence risk calculation. The improvement in RNA extraction techniques and real-time PCR platforms has led to more reproducible results, even from small biopsy samples, thus making the test more versatile across clinical scenarios. Technological innovation has also expanded the range of usable specimen types, including core needle biopsies and formalin-fixed paraffin-embedded tissues, further simplifying the logistics of test administration. As genomic databases grow and machine learning becomes more integrated into oncology research, future enhancements in recurrence scoring may also incorporate additional data such as patient demographics, comorbidities, and immune profiles for even more individualized risk assessment. In terms of accessibility, partnerships between diagnostic developers and regional pathology labs are enabling decentralized testing models, allowing quicker turnaround times and lowering the barrier to adoption in lower-resource settings. Digital pathology and cloud-based data transfer systems also facilitate remote consultation and peer review, bridging the gap between urban and rural cancer centers. The miniaturization of lab instrumentation and the advent of automated genomic analysis are making it feasible for smaller healthcare facilities to offer these tests with high accuracy. Collectively, these innovations not only ensure consistent performance across patient populations but also play a crucial role in scaling up test availability globally - making personalized breast cancer care a tangible reality for more women, regardless of geographic location.What Key Market Forces Are Driving the Expansion of Breast Recurrence Score Testing Worldwide?
The growth in the Breast Recurrence Score Test market is driven by several factors directly linked to changing cancer care protocols, technological maturity, and shifting patient expectations. A major driver is the global rise in breast cancer incidence, particularly among younger populations and in developing regions where awareness and early diagnosis are rapidly improving. This has created a strong demand for tools that can personalize treatment and avoid the physical, emotional, and financial toll of overtreatment. Concurrently, the shift toward de-escalation of therapy - especially chemotherapy - in early-stage breast cancer has positioned recurrence score tests as critical decision-making aids. Healthcare providers are increasingly seeking diagnostic tools that provide actionable insights, and the Breast Recurrence Score Test fits that role by delivering clinically validated, easy-to-interpret results that directly influence treatment pathways. Moreover, a growing population of well-informed patients is demanding transparency and precision in their care, leading oncologists to adopt genomic testing not just as a recommendation, but as a standard of care. On the provider side, multi-disciplinary tumor boards and value-based healthcare models are further promoting test usage to optimize resource utilization and outcomes. Another important market force is the active role of diagnostic companies in expanding their global footprint through strategic partnerships, awareness campaigns, and clinician education programs. These efforts are particularly impactful in Asia-Pacific and Latin American regions, where local validation studies and regulatory approvals are gaining momentum. Finally, the broader movement toward integrating genomics into routine oncology practice - from biomarker-based treatment to risk stratification - has created a fertile environment for the sustained growth of recurrence score testing. These interconnected factors - epidemiological, clinical, economic, and technological - are collectively ensuring that the global market for Breast Recurrence Score Tests remains on a robust upward trajectory.Report Scope
The report analyzes the Breast Recurrence Score Test market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Indication (Stage 1 Breast Cancer Indication, Stage 2 Breast Cancer Indication, Estrogen-Receptor-Positive Cancer Indication, Lymph-Node-Negative Cancer Indication); End-Use (Hospitals End-Use, Specialty Cancer Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Stage 1 Breast Cancer Indication segment, which is expected to reach US$158.4 Million by 2030 with a CAGR of a 10.3%. The Stage 2 Breast Cancer Indication segment is also set to grow at 9.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $67.9 Million in 2024, and China, forecasted to grow at an impressive 13.9% CAGR to reach $91.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Breast Recurrence Score Test Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Breast Recurrence Score Test Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Breast Recurrence Score Test Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as 3M, BEYA Medical, Crosstex International, Inc., EDM3 Solutions, EFELAB SRL and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Breast Recurrence Score Test market report include:
- Abbott Laboratories
- Agendia
- Biocartis
- Bio-Rad Laboratories
- Caris Life Sciences
- Epic Sciences
- Exact Sciences
- Foundation Medicine
- Guardant Health
- Hologic Inc.
- Illumina Inc.
- Myriad Genetics
- NanoString Technologies
- Natera
- OncoDNA
- Oncomatryx
- Qiagen
- Roche Diagnostics
- Thermo Fisher Scientific
- Varian Medical Systems
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Agendia
- Biocartis
- Bio-Rad Laboratories
- Caris Life Sciences
- Epic Sciences
- Exact Sciences
- Foundation Medicine
- Guardant Health
- Hologic Inc.
- Illumina Inc.
- Myriad Genetics
- NanoString Technologies
- Natera
- OncoDNA
- Oncomatryx
- Qiagen
- Roche Diagnostics
- Thermo Fisher Scientific
- Varian Medical Systems
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 282 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
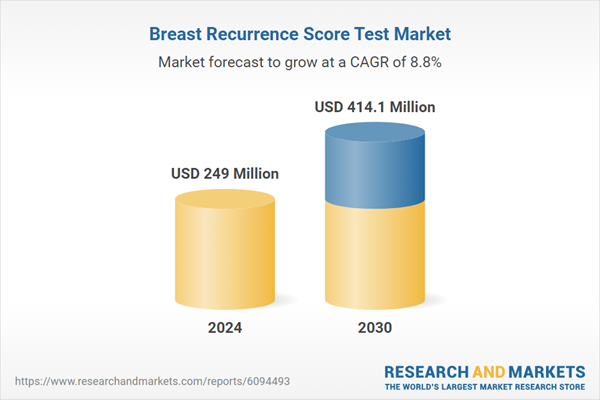
| Estimated Market Value ( USD | $ 249 Million |
| Forecasted Market Value ( USD | $ 414.1 Million |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |