Global Plasma-Derived Medicines Market - Key Trends & Drivers Summarized
Why Are Plasma-Derived Medicines Critical in Treating Rare and Chronic Disorders?
Plasma-derived medicines, obtained through fractionation of donated human plasma, play a vital role in managing a range of life-threatening and chronic conditions, including hemophilia, primary immunodeficiencies, hereditary angioedema, and autoimmune disorders. These biologics include immunoglobulins (IVIG/SCIG), clotting factors (Factor VIII, Factor IX), albumin, and hyperimmune globulins used in post-exposure prophylaxis. Unlike synthetic drugs, plasma-derived therapies are complex biologics that cannot be replaced with small-molecule alternatives, and in many cases, they remain the only therapeutic option available for patients.Their importance has grown with the increasing global prevalence of immunodeficiencies and rare bleeding disorders, particularly as diagnostic capabilities expand and newborn screening programs identify affected individuals earlier. Additionally, plasma-based therapies are being increasingly used in off-label and critical care settings, such as septic shock, Guillain-Barré syndrome, and post-transplant immunomodulation. With a growing global population living longer with chronic immune conditions, the medical reliance on plasma proteins continues to intensify, creating upward pressure on both demand and supply chains.
What Are the Technological and Operational Challenges Shaping the Supply Landscape?
The production of plasma-derived medicines is a highly specialized, resource-intensive process involving rigorous donor screening, plasma collection, pathogen inactivation, and cold-chain logistics. Fractionation, the process of separating plasma proteins, takes 7-12 months from collection to final product due to multiple purification and validation steps. This extended lead time, combined with strict biological standards and batch variability, makes the industry highly capital- and compliance-intensive. Any disruption in donor availability or facility operations can result in downstream supply bottlenecks, as witnessed during the COVID-19 pandemic when plasma donations declined significantly worldwide.Technological advances in chromatography, virus filtration, and nanofiltration are improving the yield, purity, and safety of plasma derivatives. Automated plasmapheresis systems and digital donor engagement tools are enhancing collection efficiency. However, the scalability of these advancements is constrained by the biological nature of plasma - each batch is unique, and consistent manufacturing outcomes require continuous quality control. Moreover, pathogen safety remains a top priority; regulatory mandates necessitate multiple virus inactivation steps, including solvent-detergent treatment and heat pasteurization, which add cost and complexity to every product. These operational realities have led to a global plasma supply imbalance, with developed markets heavily reliant on a few large-scale donor networks, especially in the U.S.
How Are Market Leaders and Governments Responding to Rising Global Demand?
To address growing demand, biopharmaceutical companies are investing heavily in expanding plasma collection centers, upgrading fractionation capacities, and regionalizing manufacturing to ensure better access and supply resilience. Industry leaders such as CSL Behring, Grifols, Takeda, and Octapharma are acquiring independent collection networks, building plasma banks, and integrating donor outreach with digital health platforms. These companies are also forming public-private partnerships to improve plasma access in underrepresented geographies, such as Southeast Asia, Latin America, and Eastern Europe.Governments and regulatory bodies are taking steps to safeguard national plasma supply security. Initiatives such as the European Commission's Blood and Plasma Initiative and the U.S. HHS-supported Plasma Protein Therapeutics Association (PPTA) are encouraging voluntary donation, improving reimbursement models, and incentivizing local collection infrastructure. The World Health Organization has called for the development of national self-sufficiency strategies, particularly in low- and middle-income countries, where reliance on imported products leaves patients vulnerable to shortages and price shocks. Meanwhile, emerging biotech firms are exploring recombinant alternatives and synthetic biology approaches to reduce dependency on human plasma, although such products are still in early clinical phases.
What's Powering the Long-Term Growth of the Plasma-Derived Medicines Market?
The growth in the global plasma-derived medicines market is driven by several factors, including rising prevalence of immunodeficiencies and rare hematological disorders, improved global diagnostic capabilities, and expanding off-label applications. The increasing use of IVIG in neurology and autoimmune therapies, alongside growing demand for albumin in liver disease and critical care, is significantly expanding the market beyond its traditional hematology base. Aging populations, particularly in developed countries, are also contributing to higher treatment volumes, as older patients require immunoglobulin therapies for age-associated immune decline.Furthermore, strong regulatory support for plasma safety, increasing public awareness of donation, and strategic investments by major biopharma companies are reinforcing supply chains. Growth in contract plasma fractionation services, digital donor engagement tools, and personalized therapy regimens is enabling better production planning and treatment customization. Additionally, the entry of emerging economies into the plasma collection and manufacturing space is expected to improve global accessibility and reduce regional disparities. As chronic disease management becomes more biologics-centric, and as global health systems prioritize continuity of care in rare disorders, plasma-derived medicines are poised for robust, sustained growth in the years ahead.
Report Scope
The report analyzes the Plasma-Derived Medicines market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Immunoglobulin, Coagulation Factors, Albumin, Other Products); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies); Application (Pelvic Inflammatory Disease, Bleeding Disorders, Alpha-1 Antitrypsin Deficiency, Hereditary Angioedema, Chronic Inflammatory Demyelinating Polyneuropathy, Guillain-Barre Syndrome, Multifocal Motor Neuropathy, Liver Diseases, Primary Immune Thrombocytopenia, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Immunoglobulin segment, which is expected to reach US$9.7 Billion by 2030 with a CAGR of a 4.6%. The Coagulation Factors segment is also set to grow at 7.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.9 Billion in 2024, and China, forecasted to grow at an impressive 8.8% CAGR to reach $5 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Plasma-Derived Medicines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Plasma-Derived Medicines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Plasma-Derived Medicines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AFS Medical, B. Braun Melsungen AG, Boston Scientific Corporation, Cook Medical, Dornier MedTech and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Plasma-Derived Medicines market report include:
- ADMA Biologics, Inc.
- Aurobindo Pharma Limited
- Baxter International Inc.
- Bharat Serums and Vaccines Limited
- Bio Products Laboratory Ltd (BPL)
- Biotest AG
- CSL Behring
- China Biologic Products Holdings, Inc.
- Fusion Healthcare
- Grifols, S.A.
- Hualan Biological Engineering Inc.
- Kamada Ltd.
- Kedrion Biopharma
- LFB S.A.
- Octapharma AG
- Panacea Biotec
- Sanquin
- Shanghai RAAS Blood Products Co., Ltd.
- SK Plasma
- Takeda Pharmaceutical Company Limited
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ADMA Biologics, Inc.
- Aurobindo Pharma Limited
- Baxter International Inc.
- Bharat Serums and Vaccines Limited
- Bio Products Laboratory Ltd (BPL)
- Biotest AG
- CSL Behring
- China Biologic Products Holdings, Inc.
- Fusion Healthcare
- Grifols, S.A.
- Hualan Biological Engineering Inc.
- Kamada Ltd.
- Kedrion Biopharma
- LFB S.A.
- Octapharma AG
- Panacea Biotec
- Sanquin
- Shanghai RAAS Blood Products Co., Ltd.
- SK Plasma
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 391 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
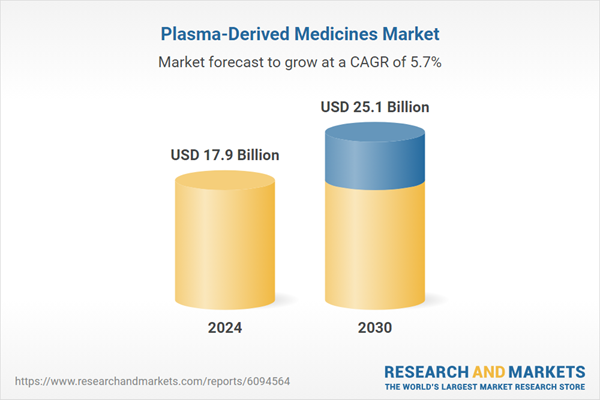
| Estimated Market Value ( USD | $ 17.9 Billion |
| Forecasted Market Value ( USD | $ 25.1 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |