Global Anaplastic Thyroid Cancer Drugs Market - Key Trends & Drivers Summarized
Why Is There a Surge in Focus on Anaplastic Thyroid Cancer Treatments Globally?
Anaplastic thyroid cancer (ATC), though rare, is among the most aggressive and lethal forms of thyroid malignancy, prompting a significant global push for effective drug development. Traditionally, treatment options for ATC have been extremely limited due to the cancer's rapid progression, resistance to conventional therapies, and tendency to metastasize early. However, the tide is shifting as researchers and pharmaceutical companies increasingly turn their attention toward this critical unmet need. Advances in molecular oncology and improved diagnostic capabilities have allowed for earlier identification and classification of ATC, fostering a more targeted approach to its management. Healthcare systems are now prioritizing rare and high-mortality cancers, leading to increased funding and faster regulatory pathways for experimental treatments. Public health institutions and private enterprises alike are recognizing the importance of investing in therapies for diseases previously deemed untreatable due to limited commercial returns.Moreover, rising awareness among clinicians and patients is accelerating the demand for novel ATC drugs. With symptoms often resembling those of other thyroid disorders, ATC has historically suffered from delayed diagnosis, but improved imaging and genetic profiling are enabling more accurate and timely detection. This has helped expand the window for therapeutic intervention and has emphasized the need for drugs capable of acting quickly and effectively. Patient advocacy groups and rare cancer foundations are also playing a stronger role, lobbying for attention and support for ATC research. In parallel, pharmaceutical companies are leveraging orphan drug incentives, which offer tax benefits and market exclusivity, to commit resources toward drug development for this rare cancer. The urgency associated with ATC, combined with scientific and policy momentum, is reshaping the therapeutic landscape and ushering in a new era of hope for patients facing this once-hopeless diagnosis.
How Are New Therapeutic Approaches Reshaping the Anaplastic Thyroid Cancer Drug Pipeline?
The drug development pipeline for anaplastic thyroid cancer is undergoing a notable transformation, driven by an expanding understanding of the genetic and molecular abnormalities that fuel the disease. Unlike conventional chemotherapy, which often fails to halt ATC progression, emerging treatments are focused on precision medicine - targeting the specific mutations and pathways that drive tumor growth. One of the major breakthroughs in recent years has been the identification of mutations in genes such as BRAF, TP53, and TERT promoter regions. These discoveries have opened the door to targeted therapies, including kinase inhibitors that can disrupt aberrant cell signaling processes. Drugs targeting BRAF V600E mutations, for instance, have shown promise in clinical trials, especially when used in combination with MEK inhibitors. These combination therapies are demonstrating superior efficacy by attacking cancer cells through multiple mechanisms simultaneously.In addition to targeted therapies, the field is witnessing a surge in immunotherapy-based approaches. Immune checkpoint inhibitors, which have revolutionized the treatment of several other cancers, are now being evaluated for their effectiveness in ATC. While the immunologically 'cold' nature of thyroid tumors has posed challenges, early-stage studies suggest that combining checkpoint inhibitors with other agents may enhance immune system recognition and response. Furthermore, advancements in next-generation sequencing are facilitating the development of personalized treatment regimens based on individual tumor profiles. Drug repurposing is also gaining traction, with several existing cancer drugs being tested for efficacy against ATC due to their activity on shared molecular pathways. These innovative strategies are gradually shifting the treatment paradigm from non-specific, palliative care to a more strategic, life-prolonging approach. As clinical trial frameworks evolve to accommodate rare and fast-moving cancers like ATC, more patients are gaining access to experimental drugs, accelerating both data collection and therapeutic breakthroughs.
What Are the Challenges and Opportunities in Expanding Access to ATC Drugs Across Regions?
Expanding global access to effective anaplastic thyroid cancer drugs presents a complex landscape of both challenges and emerging opportunities. One of the primary barriers is the high cost of development and commercialization, particularly given the small patient population and the urgency associated with disease progression. As a result, many promising treatments are initially only available in high-income countries where healthcare systems can afford innovative therapies and have the infrastructure to support expedited regulatory approvals. In contrast, low- and middle-income countries often struggle with limited access to advanced diagnostics and clinical trials, resulting in delayed or missed diagnoses and reduced availability of cutting-edge drugs. Even when novel treatments are available, the cost of targeted therapies and immunotherapy can be prohibitive without robust insurance or public healthcare subsidies.However, international collaboration is beginning to address some of these disparities. Global consortia of researchers, oncologists, and policymakers are working together to harmonize clinical guidelines and share data across borders, making it easier to identify effective treatment strategies and replicate successes in diverse populations. Additionally, regulatory agencies are increasingly cooperating on accelerated approval pathways for rare disease therapies, which can help streamline the entry of ATC drugs into emerging markets. Non-governmental organizations and rare cancer alliances are also stepping in to provide patient assistance programs, drug access initiatives, and educational outreach that enable more equitable care delivery. Meanwhile, pharmaceutical companies are being encouraged - and in some cases mandated - to implement tiered pricing models and compassionate use programs. These developments are not only improving access but are also fostering a more inclusive research ecosystem where underrepresented patient groups are part of the drug development narrative.
What Key Factors Are Driving the Growth of the Anaplastic Thyroid Cancer Drugs Market?
The growth in the anaplastic thyroid cancer drugs market is driven by several key factors anchored in scientific advancement, changing regulatory frameworks, and evolving treatment philosophies. One of the most significant drivers is the rapid progress in molecular biology, which has transformed ATC from an untreatable diagnosis into a genetically defined target for intervention. Precision oncology tools, such as tumor genomic profiling and liquid biopsies, have become integral in guiding treatment decisions, enabling oncologists to select the most appropriate drug combinations based on mutation status. This has led to the rise of targeted therapies that not only improve outcomes but also reduce systemic toxicity compared to traditional chemotherapy. Simultaneously, the increasing inclusion of ATC patients in clinical trials for broader thyroid cancer studies is expanding the knowledge base and treatment options available.Regulatory bodies are also playing a pivotal role in stimulating market growth. Orphan drug designations, fast-track approvals, and expanded access programs are incentivizing pharmaceutical companies to invest in drug development for ATC. These regulatory advantages reduce time-to-market and provide extended exclusivity, making the segment more commercially viable despite its niche size. Additionally, the growing availability of real-world evidence from digital health records and cancer registries is helping validate the effectiveness of emerging therapies, encouraging broader adoption. From a healthcare delivery standpoint, the centralization of cancer care into multidisciplinary centers is ensuring more consistent access to expert opinion and experimental treatments. Patient-driven demand for personalized medicine, coupled with rising global awareness about rare and aggressive cancers, is also pushing healthcare systems to prioritize ATC treatment development. Altogether, these factors are converging to accelerate innovation and access in the ATC drugs market, providing new hope to patients where few options once existed.
Report Scope
The report analyzes the Anaplastic Thyroid Cancer Drugs market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Chemotherapy, Novel Therapy); End-Use (Hospitals End-Use, Clinics End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Chemotherapy segment, which is expected to reach US$912.1 Million by 2030 with a CAGR of a 6.9%. The Novel Therapy segment is also set to grow at 4.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $256.4 Million in 2024, and China, forecasted to grow at an impressive 5.8% CAGR to reach $221.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Anaplastic Thyroid Cancer Drugs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Anaplastic Thyroid Cancer Drugs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Anaplastic Thyroid Cancer Drugs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABB Ltd., Acromag Inc., Advantech Co., Ltd., AMETEK, Inc., Analog Devices, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Anaplastic Thyroid Cancer Drugs market report include:
- AbbVie Inc.
- AffyImmune Therapeutics, Inc.
- Amgen Inc.
- AstraZeneca PLC
- Bayer AG
- Bristol-Myers Squibb Company
- Daiichi Sankyo Company, Limited
- Eisai Co., Ltd.
- Eli Lilly and Company
- Exelixis, Inc.
- Genentech, Inc.
- Hikma Pharmaceuticals PLC
- Merck & Co., Inc.
- Millennium Pharmaceuticals, Inc.
- Novartis AG
- Pfizer Inc.
- Plexxikon Inc.
- Sanofi S.A.
- Seagen Inc.
- Takeda Pharmaceutical Company
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- AffyImmune Therapeutics, Inc.
- Amgen Inc.
- AstraZeneca PLC
- Bayer AG
- Bristol-Myers Squibb Company
- Daiichi Sankyo Company, Limited
- Eisai Co., Ltd.
- Eli Lilly and Company
- Exelixis, Inc.
- Genentech, Inc.
- Hikma Pharmaceuticals PLC
- Merck & Co., Inc.
- Millennium Pharmaceuticals, Inc.
- Novartis AG
- Pfizer Inc.
- Plexxikon Inc.
- Sanofi S.A.
- Seagen Inc.
- Takeda Pharmaceutical Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 142 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
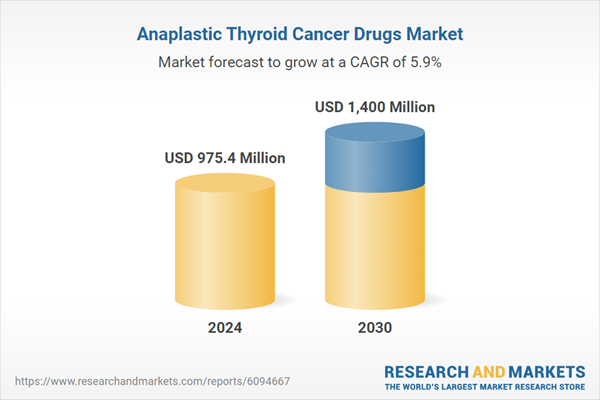
| Estimated Market Value ( USD | $ 975.4 Million |
| Forecasted Market Value ( USD | $ 1400 Million |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |