Global Blood Plasma Market - Key Trends & Drivers Summarized
Why Is Blood Plasma Considered a Critical Component in Modern Medicine?
Blood plasma, the straw-colored liquid portion of blood, plays a pivotal role in modern medicine due to its rich composition of proteins, antibodies, clotting factors, and electrolytes. Unlike whole blood, which is primarily used for transfusions in trauma cases, plasma is valued for its therapeutic properties and is processed into a wide range of life-saving treatments. These include immunoglobulins for immune deficiencies, albumin for volume expansion in critically ill patients, and clotting factors for individuals with hemophilia and other bleeding disorders. Plasma is also used in treating rare, chronic, and genetic conditions that would otherwise have few viable treatment options. In the wake of rising chronic illnesses, immune system disorders, and global public health emergencies, plasma-derived therapies are becoming more critical than ever. During the COVID-19 pandemic, for example, convalescent plasma drew attention for its potential to transfer antibodies from recovered patients to those battling the virus, highlighting the adaptability and importance of plasma in responding to emerging health threats. Furthermore, the unique nature of plasma - being both a renewable biological resource and a foundational ingredient for biologics - has made it an essential focus for pharmaceutical companies and healthcare providers. The global need for plasma continues to grow, placing immense importance on collection, processing, and equitable distribution systems. As therapeutic applications broaden and demand intensifies, blood plasma is solidifying its status as an irreplaceable pillar of both emergency care and long-term disease management.How Are Advancements in Plasma Fractionation and Processing Transforming the Industry?
Technological innovations in plasma fractionation - the process by which different therapeutic components are separated from raw plasma - are dramatically enhancing the efficiency, scalability, and safety of plasma-based therapies. Over the years, traditional cold ethanol fractionation methods have evolved into highly specialized, multi-step processes that enable precise extraction of various plasma proteins, including immunoglobulins, albumin, and coagulation factors. Newer techniques such as chromatography and nanofiltration have further increased purity levels while minimizing the risk of viral contamination, improving both patient outcomes and regulatory compliance. These advancements are enabling manufacturers to optimize yield, reduce production costs, and accelerate turnaround times, which is crucial given the global shortage of plasma and increasing demand for its derivatives. Automation and digital tracking are also streamlining quality control, traceability, and inventory management, making the entire value chain more efficient and transparent. In addition to technological progress, collaborations between biopharmaceutical companies and academic research institutions are spurring innovation in developing next-generation plasma-derived therapies that can target a broader range of diseases. Personalized medicine is also becoming a factor, with research into how specific plasma proteins can be tailored or enhanced to suit individual patient needs. As these technologies mature, they are expected to open new possibilities in treating autoimmune disorders, neurological conditions, and rare diseases. Collectively, advancements in processing and production are making plasma therapies more accessible and safer, laying the groundwork for a more robust and responsive global plasma industry.How Are Global Donation Practices and Ethical Considerations Impacting Supply Chains?
The global blood plasma supply chain is heavily dependent on human donors, making ethical collection practices, donor compensation models, and donation infrastructure pivotal to ensuring a stable and sustainable plasma supply. Currently, the United States dominates global plasma collection, accounting for more than 60% of the total global supply, largely due to its allowance of paid plasma donations - a policy not universally accepted. In contrast, many European countries restrict or prohibit monetary compensation, citing ethical concerns and donor safety, resulting in comparatively lower collection volumes. This has led to a significant supply imbalance, with many countries relying on imported plasma to meet domestic therapeutic needs. These disparities are sparking international debate about best practices for donor recruitment and the morality of commodifying a biological resource. Regulatory bodies such as the World Health Organization (WHO) and the European Medicines Agency (EMA) continue to advocate for voluntary, non-remunerated donations, but the growing global demand for plasma therapies is putting pressure on these principles. To address this challenge, several nations are investing in domestic collection infrastructure, awareness campaigns, and partnerships with nonprofit and private collection agencies. Logistics and cold-chain management also play a vital role, as plasma must be collected, transported, and processed under stringent temperature and timing requirements. Meanwhile, donor safety, transparency, and ethical sourcing remain top concerns for both governments and consumers. Striking a balance between supply chain efficiency, donor rights, and ethical standards is a complex but crucial component of the plasma industry's future stability.What Are the Key Factors Driving Growth in the Blood Plasma Market Worldwide?
The growth in the blood plasma market is driven by several interlinked factors spanning demographic trends, technological advancements, healthcare policy changes, and rising global disease burdens. One of the most influential drivers is the growing incidence of chronic and rare diseases that rely on plasma-derived therapies for effective management, including immunodeficiencies, hemophilia, and hereditary angioedema. Aging populations in developed countries are also fueling demand, as older individuals are more likely to suffer from immune dysfunction, surgical recovery needs, and critical illnesses that require plasma products. Additionally, increasing awareness and diagnosis of rare diseases, supported by improved access to specialized healthcare, are expanding the patient pool in need of these therapies. On the technological side, improvements in plasma collection, processing, and storage are enabling manufacturers to scale operations more efficiently, thus meeting growing demand more rapidly. Government policies encouraging domestic plasma collection and public-private partnerships are also playing a role, particularly in countries that historically relied on imports. Furthermore, strong investment from biopharmaceutical companies and a surge in clinical research for plasma-derived treatments are expanding the product pipeline and therapeutic applications. Market growth is also bolstered by evolving reimbursement structures, regulatory approvals, and enhanced global healthcare infrastructure, particularly in emerging economies. Together, these drivers are creating a fertile landscape for continued expansion in the plasma market, underscoring the critical role that plasma-derived products play in the evolving landscape of modern, precision-driven, and life-saving healthcare.Report Scope
The report analyzes the Blood Plasma market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Source (Human Plasma Source, Synthetic Plasma Source); Application (Therapeutic Use Application, Diagnostic Use Application, Research & Development Application).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Human Plasma Source segment, which is expected to reach US$35.7 Billion by 2030 with a CAGR of a 9.2%. The Synthetic Plasma Source segment is also set to grow at 5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $9 Billion in 2024, and China, forecasted to grow at an impressive 12.4% CAGR to reach $11.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Blood Plasma Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Blood Plasma Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Blood Plasma Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AirBands, B3 Sciences, BFR Bands / Exerscribe Inc., B STRONG, Delfi Medical Innovations Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Blood Plasma market report include:
- ADMA Biologics Inc.
- Baxter International Inc.
- Bio Products Laboratory Ltd (BPL)
- Biotest AG
- Canadian Blood Services
- Canadian Plasma Resources
- Cerus Corporation
- China Biologic Products Holdings
- CSL Behring
- Emergent BioSolutions Inc.
- Grifols S.A.
- Green Cross Corporation
- Hualan Biological Engineering Inc.
- Intas Pharmaceuticals Ltd.
- Kamada Ltd.
- Kedrion Biopharma
- LFB S.A.
- Octapharma AG
- Sanquin Blood Supply Foundation
- Takeda Pharmaceutical Company Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ADMA Biologics Inc.
- Baxter International Inc.
- Bio Products Laboratory Ltd (BPL)
- Biotest AG
- Canadian Blood Services
- Canadian Plasma Resources
- Cerus Corporation
- China Biologic Products Holdings
- CSL Behring
- Emergent BioSolutions Inc.
- Grifols S.A.
- Green Cross Corporation
- Hualan Biological Engineering Inc.
- Intas Pharmaceuticals Ltd.
- Kamada Ltd.
- Kedrion Biopharma
- LFB S.A.
- Octapharma AG
- Sanquin Blood Supply Foundation
- Takeda Pharmaceutical Company Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 282 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
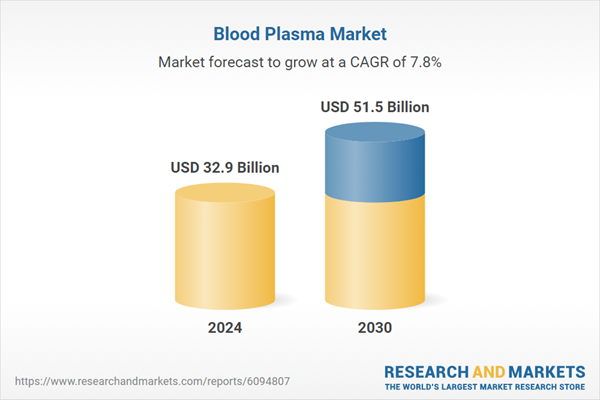
| Estimated Market Value ( USD | $ 32.9 Billion |
| Forecasted Market Value ( USD | $ 51.5 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |