Global 'Gene Delivery Technologies' Market - Key Trends & Drivers Summarized
Why Are Gene Delivery Technologies at the Core of Modern Therapeutics and Genomic Medicine?
Gene delivery technologies are revolutionizing the landscape of biomedical research and clinical treatment, serving as the fundamental enablers for gene therapy, genome editing, and personalized medicine. These technologies facilitate the safe and efficient introduction of genetic material - DNA, RNA, or CRISPR-Cas systems - into target cells, a process essential for correcting genetic defects, modulating gene expression, or creating disease models. With the surge in interest surrounding genetic diseases, cancers, and viral infections, the demand for reliable gene delivery platforms has skyrocketed. Unlike traditional pharmaceuticals, gene-based interventions require precise and targeted delivery to be effective and safe, thus elevating the importance of both viral vectors (like adeno-associated virus, lentivirus, and retrovirus) and non-viral methods (such as lipid nanoparticles, electroporation, and polymer-based systems). The recent success of mRNA vaccines for COVID-19, which relied on lipid-based delivery, has further validated the commercial and therapeutic viability of gene delivery tools, sparking unprecedented research funding and cross-industry collaborations.How Are Emerging Technologies Enhancing Safety, Precision, and Scalability in Gene Delivery?
Technological innovation is dramatically advancing the capabilities of gene delivery systems, addressing past limitations in immunogenicity, transfection efficiency, and tissue specificity. In viral vectors, new-generation adeno-associated viruses (AAVs) with engineered capsids are offering improved tropism and reduced immune responses, making them safer for systemic applications in diseases like hemophilia, retinal dystrophies, and spinal muscular atrophy. Lentiviral vectors, known for their integration into host genomes, are being optimized for stem cell and CAR-T cell therapies. On the non-viral front, lipid nanoparticles (LNPs) have surged to the forefront, especially in mRNA-based therapies, offering high efficiency, modular design, and low toxicity. Electroporation and microfluidic-based systems are gaining traction for ex vivo applications, particularly in autologous cell therapies. Additionally, smart delivery platforms responsive to pH, enzymes, or external triggers (e.g., light or ultrasound) are being explored to improve site-specific gene release. These innovations not only enhance therapeutic outcomes but also support large-scale, GMP-compliant manufacturing - critical for clinical and commercial deployment.What Role Do Clinical Pipelines, Regulatory Trends, and Industry Investment Play?
The rapid expansion of gene therapy clinical pipelines across oncology, neurology, and rare diseases is a key catalyst for the gene delivery technologies market. Dozens of gene-based therapies are in advanced stages of clinical development, with regulatory bodies such as the U.S. FDA and EMA accelerating review pathways for breakthrough designations. This regulatory momentum is encouraging startups, biotech firms, and pharma giants alike to intensify investment in delivery platforms that ensure safety, reproducibility, and long-term efficacy. Mergers, licensing deals, and R&D collaborations between delivery tech developers and therapeutic companies have spiked, signaling a growing recognition of delivery systems as strategic assets rather than technical afterthoughts. Additionally, regulatory guidance is now increasingly emphasizing vector characterization, biodistribution, and immune profiling - areas directly tied to delivery mechanism design. Meanwhile, public and private funding initiatives, including national genomics missions and disease-specific foundations, are supporting delivery-focused research to address underserved diseases and populations, further expanding global market opportunities.What Are the Key Growth Drivers Fueling the Global Expansion of Gene Delivery Technologies?
The growth in the global gene delivery technologies market is driven by several interconnected factors stemming from clinical innovation, therapeutic demand, and biotechnological advancement. Foremost is the increasing number of gene therapy trials and approvals, which inherently require robust and scalable delivery mechanisms tailored to specific disease targets and patient populations. Second, the broadening range of gene therapy indications - from monogenic disorders to complex cancers and infectious diseases - is necessitating the development of diverse and adaptable delivery platforms. Third, advances in synthetic biology and genome engineering are expanding the need for precision delivery systems that can accommodate larger payloads, multiplex gene edits, and tightly regulated expression profiles. Fourth, rising investment from both governments and the private sector in genetic medicine is accelerating platform development and commercialization pathways. Lastly, the success of mRNA-based therapeutics and vaccines has demonstrated the market potential for non-viral delivery systems, spurring further innovation in nanoparticle engineering, polymer science, and conjugation chemistry. These factors collectively underpin the robust global expansion of gene delivery technologies as a foundational pillar of next-generation medicine.Report Scope
The report analyzes the Gene Delivery Technologies market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Mode (Biological Mode, Chemical Mode, Physical Mode); Method (Ex Vivo Method, In Vivo Method, In Vitro Method); Application (Gene Therapy Application, Cell Therapy Application, Vaccines Application, Research Application).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 34 companies featured in this Gene Delivery Technologies market report include -
- Alnylam Pharmaceuticals Inc.
- Amgen Inc.
- American Gene Technologies
- AskBio (subsidiary of Bayer AG)
- Astellas Gene Therapies
- Beam Therapeutics Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- BioNTech SE
- Bluebird Bio Inc.
- Catalent, Inc.
- CRISPR Therapeutics AG
- Editas Medicine Inc.
- ElevateBio
- F. Hoffmann-La Roche AG
- Genentech, Inc.
- GenScript ProBio
- Horizon Discovery Ltd.
- Intellia Therapeutics Inc.
- Johnson & Johnson
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Biological Mode segment, which is expected to reach US$3.2 Billion by 2030 with a CAGR of a 10.3%. The Chemical Mode segment is also set to grow at 5.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $805.5 Million in 2024, and China, forecasted to grow at an impressive 13.9% CAGR to reach $1.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Gene Delivery Technologies Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Gene Delivery Technologies Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Gene Delivery Technologies Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Acelity Inc., Alimed Inc., American Medical Depot, B. Braun Melsungen AG, Burlington Medical and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 34 Featured):
- Alnylam Pharmaceuticals Inc.
- Amgen Inc.
- American Gene Technologies
- AskBio (subsidiary of Bayer AG)
- Astellas Gene Therapies
- Beam Therapeutics Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- BioNTech SE
- Bluebird Bio Inc.
- Catalent, Inc.
- CRISPR Therapeutics AG
- Editas Medicine Inc.
- ElevateBio
- F. Hoffmann-La Roche AG
- Genentech, Inc.
- GenScript ProBio
- Horizon Discovery Ltd.
- Intellia Therapeutics Inc.
- Johnson & Johnson
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alnylam Pharmaceuticals Inc.
- Amgen Inc.
- American Gene Technologies
- AskBio (subsidiary of Bayer AG)
- Astellas Gene Therapies
- Beam Therapeutics Inc.
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- BioNTech SE
- Bluebird Bio Inc.
- Catalent, Inc.
- CRISPR Therapeutics AG
- Editas Medicine Inc.
- ElevateBio
- F. Hoffmann-La Roche AG
- Genentech, Inc.
- GenScript ProBio
- Horizon Discovery Ltd.
- Intellia Therapeutics Inc.
- Johnson & Johnson
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 370 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
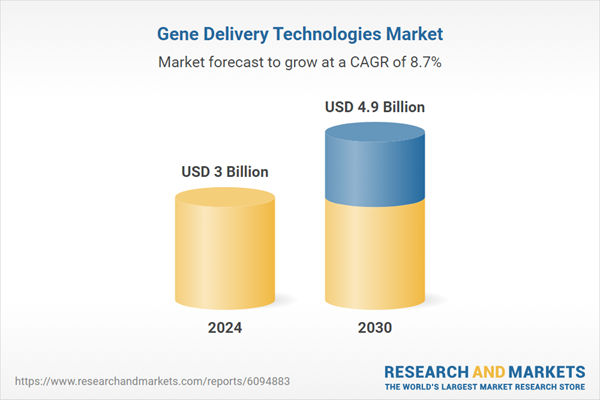
| Estimated Market Value ( USD | $ 3 Billion |
| Forecasted Market Value ( USD | $ 4.9 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |