Global Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Market - Key Trends & Drivers Summarized
How Is the Therapeutic Landscape for DLBCL Evolving Amid Rising Clinical Complexity?
Diffuse Large B-Cell Lymphoma (DLBCL) represents the most common and aggressive subtype of non-Hodgkin lymphoma. The therapeutic landscape is undergoing a paradigm shift, driven by the need to address disease heterogeneity and improve long-term survival outcomes. While the R-CHOP regimen remains the first-line standard, nearly one-third of patients relapse or develop resistance, creating an urgent need for next-generation therapies. Subtyping of DLBCL into germinal center B-cell (GCB) and activated B-cell (ABC) categories, based on gene expression profiles, has laid the foundation for targeted treatment approaches.The market is steadily transitioning from generalized chemotherapeutic strategies to precision therapeutics tailored to molecular and immunological subtypes. Biomarker testing, immune cell profiling, and genetic mutation analysis are becoming integral to therapy selection. There is growing emphasis on developing therapies that not only extend progression-free survival but also offer better quality of life through reduced toxicity, outpatient administration, and faster remission onset. The rapid approval pace of novel drug classes is reflective of this shift toward a more sophisticated and targeted therapeutic model.
Which Therapeutic Innovations Are Reshaping the DLBCL Treatment Paradigm?
Immunotherapies and precision-targeted agents are at the forefront of innovation in DLBCL therapeutics. Anti-CD20 monoclonal antibodies such as obinutuzumab and biosimilars of rituximab are expanding the options for first-line treatment. For relapsed and refractory cases, CAR-T cell therapies - especially CD19-directed variants - are showing high response durability in otherwise treatment-resistant patients. The market is also witnessing the emergence of bispecific T-cell engagers and antibody-drug conjugates that offer dual-targeting mechanisms for improved cytotoxicity.In addition to immune-based therapies, small-molecule inhibitors targeting BCL2, BTK, and EZH2 are being developed for specific genetic subgroups, offering hope for patients with poor prognostic indicators. These therapies are often used in combination with immunochemotherapy to improve efficacy and overcome resistance. There is also increasing interest in checkpoint inhibitors and novel cytokine-based therapies, which aim to modulate the tumor microenvironment. With over a hundred clinical trials underway globally, the pipeline remains robust and diversified.
How Are End-Use Settings and Treatment Demographics Influencing Market Demand?
The demand for DLBCL therapeutics is being shaped by trends in healthcare access, population aging, and the stratification of patient groups based on disease biology and treatment history. Elderly patients, often ineligible for aggressive chemotherapy, are driving demand for safer, low-toxicity regimens that can be administered in outpatient or home-care settings. Meanwhile, younger patients with relapsed disease are increasingly turning to high-cost, high-promise therapies like CAR-T, supported by expanding reimbursement models in advanced economies.Hospitals and specialty cancer centers remain the primary treatment venues for complex regimens involving immunotherapy and cell-based products. However, community clinics are beginning to adopt biosimilars and targeted agents due to their simplified administration and expanding availability. Precision medicine tools are also being integrated into diagnostic labs to help guide frontline therapy decisions. Academic institutions are leading in trial enrollment and translational research, playing a critical role in bridging innovation and clinical practice.
What Factors Are Driving the Growth of the DLBCL Therapeutics Market?
The growth in the diffuse large B-cell lymphoma therapeutics market is driven by several factors, including rising diagnosis rates due to improved screening tools, expanding use of immunotherapy in both frontline and salvage settings, and continuous innovation in biomarker-driven treatment protocols. The increasing number of patients with relapsed or refractory disease is creating sustained demand for novel agents that can provide durable responses beyond first-line chemotherapy.Technological advancements in CAR-T cell manufacturing, enhancements in next-generation sequencing for molecular profiling, and increasing penetration of biosimilar monoclonal antibodies are accelerating therapy adoption across regions. The growing availability of targeted therapies tailored to genetic mutations and cell-of-origin classification is also enhancing clinical outcomes. Additionally, favorable regulatory pathways, rising healthcare expenditure in emerging markets, and evolving payer support for high-cost cell and gene therapies are collectively fueling the expansion of the global DLBCL therapeutics market.
Report Scope
The report analyzes the Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Small Molecules, Biologics); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Distribution Channel, Other Distribution Channels).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Small Molecules segment, which is expected to reach US$5.2 Billion by 2030 with a CAGR of a 8.5%. The Biologics segment is also set to grow at 4.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.4 Billion in 2024, and China, forecasted to grow at an impressive 11.4% CAGR to reach $1.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AGC Inc., Apollo Optical Systems Inc., Broadcom Inc., Coherent Corp., Edmund Optics Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Diffuse Large B-Cell Lymphoma (DLBCL) Therapeutics market report include:
- AbbVie Inc.
- ADC Therapeutics SA
- Amgen Inc.
- Bayer AG
- BeiGene Ltd.
- Bristol Myers Squibb Co.
- Celltrion Healthcare Co. Ltd.
- CTI BioPharma Corp.
- Erytech Pharma SA
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences Inc.
- GlaxoSmithKline Plc
- Johnson & Johnson Services Inc.
- Merck & Co. Inc.
- Novartis AG
- Pfizer Inc.
- Seagen Inc.
- Spectrum Pharmaceuticals Inc.
- Takeda Pharmaceutical Co. Ltd.
- Teva Pharmaceutical Industries Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- ADC Therapeutics SA
- Amgen Inc.
- Bayer AG
- BeiGene Ltd.
- Bristol Myers Squibb Co.
- Celltrion Healthcare Co. Ltd.
- CTI BioPharma Corp.
- Erytech Pharma SA
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences Inc.
- GlaxoSmithKline Plc
- Johnson & Johnson Services Inc.
- Merck & Co. Inc.
- Novartis AG
- Pfizer Inc.
- Seagen Inc.
- Spectrum Pharmaceuticals Inc.
- Takeda Pharmaceutical Co. Ltd.
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
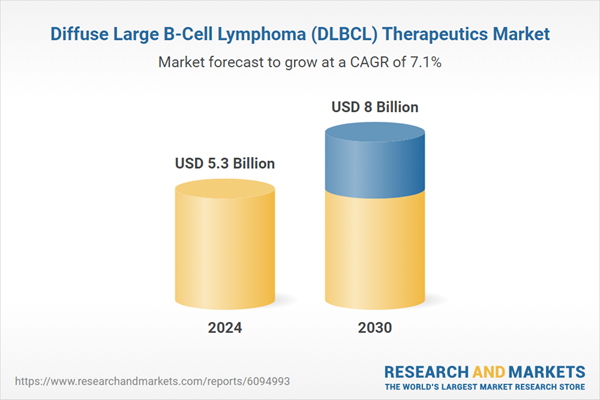
| No. of Pages | 273 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 5.3 Billion |
| Forecasted Market Value ( USD | $ 8 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |