Global Endobronchial Valves Market - Key Trends & Drivers Summarized
How Are Technological Innovations Reshaping Endobronchial Valve Design and Deployment?
Endobronchial valves (EBVs), as one-way, implantable bronchoscopic devices, have undergone transformative advancements in design and deployment techniques over the last decade. These valves are predominantly used in minimally invasive treatment of severe emphysema and persistent air leaks, offering significant anatomical and functional improvements in lung volume reduction (BLVR). Traditionally, the challenge has been optimizing the precision of valve placement within the heterogeneous and often hyperinflated lobes of the lungs. Innovations such as customizable sizes, advanced anchoring mechanisms, and repositionable deployment features have greatly improved procedural outcomes and reduced the rate of valve migration or occlusion. Companies have introduced valves with nitinol frames and silicone membranes that conform more effectively to bronchial anatomy while preserving durability and biocompatibility.Imaging-guided deployment has further elevated the clinical precision of EBVs. Integration with advanced diagnostic modalities like high-resolution computed tomography (HRCT) and digital bronchoscopic navigation platforms enables pulmonologists to pre-assess fissure completeness, identify optimal target lobes, and predict collateral ventilation. This imaging support is crucial because incomplete fissures or collateral ventilation reduces the therapeutic efficacy of EBVs. As a result, the focus is increasingly on patient-specific planning aided by artificial intelligence (AI)-enabled diagnostic platforms, which are now being incorporated into the valve placement workflow. Furthermore, improvements in catheter delivery systems have reduced deployment time, minimized procedural trauma, and made outpatient procedures more feasible, thereby expanding the candidate pool and reducing hospitalization rates.
Where Are Endobronchial Valves Gaining Most Traction in Clinical Use?
Clinical applications for endobronchial valves are expanding beyond traditional emphysema-related indications. The predominant market remains chronic obstructive pulmonary disease (COPD), particularly in cases of severe heterogeneous emphysema not amenable to surgical interventions. Within this segment, a large proportion of patients suffer from upper-lobe predominant disease patterns, for which EBVs have shown consistent results in improving lung function, exercise capacity, and quality of life. However, their adoption is also accelerating in cases of persistent air leaks, post-lobectomy complications, and pneumothorax management, particularly in intensive care and post-operative units. In such settings, EBVs are being considered as salvage therapies where conventional chest tube management proves ineffective.The procedural uptake is notably increasing across North America and Western Europe, where reimbursement pathways have been clarified for bronchoscopic lung volume reduction (BLVR). The clinical endorsement of EBVs by regulatory agencies such as the U.S. FDA and their inclusion in global GOLD (Global Initiative for Chronic Obstructive Lung Disease) treatment guidelines have also legitimized their role within interventional pulmonology. Another emerging trend is the adoption of EBVs in Asia-Pacific countries, particularly Japan and South Korea, which are seeing a rise in COPD incidence due to aging populations and changing air quality indices. In these regions, local manufacturers are entering licensing partnerships or co-developing valves tailored for regional anatomical profiles, further accelerating localized acceptance and penetration into tertiary care hospitals and specialty clinics.
What Market Forces Are Steering R&D, Regulatory, and Patient Adoption Dynamics?
The commercialization of endobronchial valves is significantly influenced by multiple dynamic forces, particularly regulatory evolution, payer acceptance, and surgeon training programs. The regulatory landscape has gradually matured in developed markets, with major products receiving CE mark and PMA (Premarket Approval) status. However, product adoption still hinges on structured physician training and credentialing programs, as the procedure requires meticulous patient selection and bronchoscopic expertise. Leading manufacturers are investing in simulator-based training modules and workshops to reduce the learning curve, which in turn supports wider physician adoption and standardization across pulmonary centers.Patient behavior is also shaping the market trajectory. Increased health literacy and the shift toward minimally invasive procedures with rapid recovery cycles are pushing patients to seek non-surgical alternatives to lung volume reduction surgery (LVRS). Endobronchial valves fulfill this demand, especially among patients who are deemed inoperable or at high surgical risk. Furthermore, the inclusion of EBVs under private insurance and national health reimbursement programs - especially in Germany, the UK, and selected U.S. states - has improved treatment affordability and accessibility. Nevertheless, pricing remains a sensitive factor, particularly in public hospitals and emerging economies. As competitive intensity increases, mid-tier companies are developing cost-optimized valves to address price-sensitive regions without compromising on clinical outcomes.
Simultaneously, clinical evidence is reinforcing the market. Longitudinal studies such as the LIBERATE and STELVIO trials have showcased durable improvements in forced expiratory volume (FEV1) and reductions in hyperinflation among patients implanted with EBVs. This growing corpus of real-world evidence is enhancing physician confidence and justifying health system investments into endobronchial technologies. Finally, strategic partnerships between medtech firms and pulmonary research institutions are boosting pipeline innovation, leading to next-generation valves with better integration, biodegradability, and remote monitoring potential. These emerging technologies may eventually support closed-loop respiratory systems in chronic care settings.
What Is Driving the Accelerated Growth of the Endobronchial Valves Market?
The growth in the global endobronchial valves market is driven by several factors that collectively strengthen its value proposition across pulmonology, critical care, and minimally invasive therapy domains. A primary growth catalyst is the rising global burden of COPD and emphysema, especially in aging populations and regions with high tobacco use or environmental pollution. These demographics create a large pool of patients with advanced-stage lung disease who are not suitable candidates for surgical resection, thereby necessitating bronchoscopic alternatives like EBVs. As the incidence of such conditions continues to rise, so too does the demand for non-surgical interventions that provide measurable symptomatic relief with a favorable safety profile.Technological differentiation is another major driver. Companies that offer valves with enhanced deliverability, improved sealing properties, and long-term patency are outpacing competitors in market share gains. The presence of integrated imaging and AI-aided lung segmentation tools further raises the confidence levels of interventional pulmonologists, helping drive procedural volume growth across both academic centers and private clinics. Simultaneously, increased funding in respiratory research and clinical trials by governments and private foundations is helping validate newer indications, such as air leak management and bronchopleural fistulae treatment, thereby widening the scope of valve applicability.
Healthcare infrastructure and economic enablers also play an essential role. Reimbursement clarity in key markets such as the U.S., Germany, and the UK has eliminated a key adoption barrier. Additionally, strategic pricing models and value-based procurement mechanisms are making EBVs more accessible in developing healthcare systems across Latin America and Southeast Asia. Training and educational outreach programs are equipping pulmonologists with the necessary procedural skillsets, accelerating institutional approvals and inclusion in hospital formularies. Moreover, the ecosystem is maturing with stronger aftercare protocols, post-implantation monitoring systems, and standardized follow-up practices that support long-term patient outcomes. These combined forces ensure that the endobronchial valves market is positioned for sustained growth over the forecast period, marked by continual clinical expansion and deeper global penetration.
Report Scope
The report analyzes the Endobronchial Valves market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Duckbill-Shaped Valves, Umbrella-Shaped Valves); End-Use (Hospitals End-Use, Clinical Research Center End-Use, Ambulatory Surgery Center End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Duckbill-Shaped Valves segment, which is expected to reach US$146.7 Million by 2030 with a CAGR of a 5.6%. The Umbrella-Shaped Valves segment is also set to grow at 3.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $47.9 Million in 2024, and China, forecasted to grow at an impressive 8.6% CAGR to reach $48.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Endobronchial Valves Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Endobronchial Valves Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Endobronchial Valves Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ANCA Pty Ltd, CERATIZIT Group, Dormer Pramet, Emuge Corporation, Garr Tool Company and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Endobronchial Valves market report include:
- ALung Technologies Inc.
- Boston Scientific Corporation
- Bracco Imaging S.p.A.
- Broncus Medical Inc.
- BTG International Ltd.
- Cook Medical
- Endo-Flex GmbH
- Ethicon US LLC
- Harrington Medical
- Innovative Airway Solutions
- MedLung
- Medtronic plc
- Merit Medical Systems Inc.
- Novatech SA
- Olympus Corporation
- Pulmonx Corporation
- Spiration Inc.
- Teleflex Incorporated
- VAPotherm Inc.
- Vyaire Medical Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ALung Technologies Inc.
- Boston Scientific Corporation
- Bracco Imaging S.p.A.
- Broncus Medical Inc.
- BTG International Ltd.
- Cook Medical
- Endo-Flex GmbH
- Ethicon US LLC
- Harrington Medical
- Innovative Airway Solutions
- MedLung
- Medtronic plc
- Merit Medical Systems Inc.
- Novatech SA
- Olympus Corporation
- Pulmonx Corporation
- Spiration Inc.
- Teleflex Incorporated
- VAPotherm Inc.
- Vyaire Medical Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 280 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
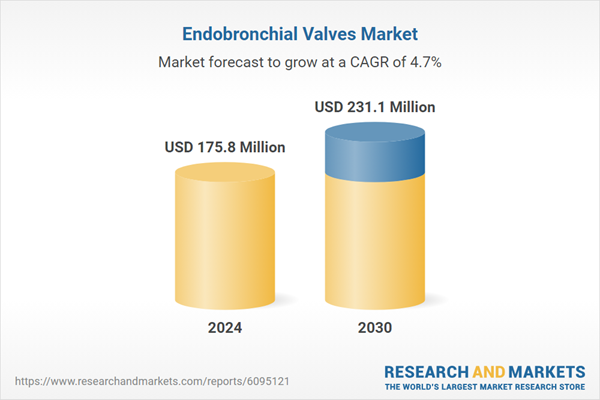
| Estimated Market Value ( USD | $ 175.8 Million |
| Forecasted Market Value ( USD | $ 231.1 Million |
| Compound Annual Growth Rate | 4.7% |
| Regions Covered | Global |