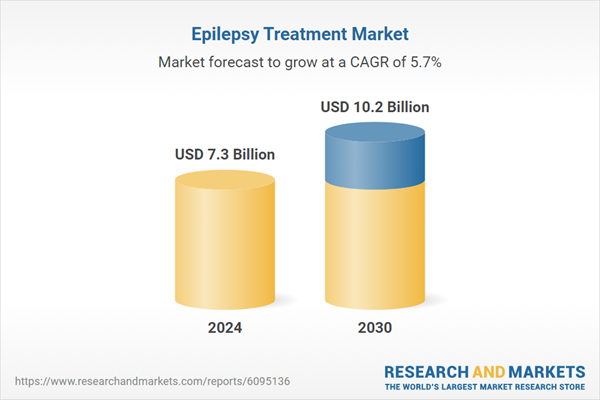
Global Epilepsy Treatment Market - Key Trends & Drivers Summarized
How Is Innovation in Drug Development Rewriting the Rules of Epilepsy Care?
The pharmaceutical landscape for epilepsy treatment is rapidly evolving, driven by the limitations of traditional antiepileptic drugs (AEDs) and a growing emphasis on personalized and precision therapies. Although older-generation AEDs such as carbamazepine, phenytoin, and valproate remain staples in clinical settings, their long-term side effects, teratogenic risks, and metabolic complications have prompted the search for more targeted interventions. The market is witnessing the entry of next-generation AEDs like brivaracetam, perampanel, and cenobamate that offer improved seizure control, fewer drug-drug interactions, and better tolerability. These agents are increasingly being positioned as first-line therapies for focal and generalized seizures, particularly in newly diagnosed patients.In parallel, there is a growing momentum behind therapies targeting specific genetic mutations linked to treatment-resistant epilepsy syndromes such as Dravet syndrome and Lennox-Gastaut syndrome. Precision drug discovery efforts have led to the development of cannabidiol-based therapies like Epidiolex, which has seen strong uptake due to its favorable seizure reduction profile and regulatory support in multiple countries. Meanwhile, pipeline candidates such as soticlestat and ganaxolone are under advanced stages of clinical evaluation, with promising efficacy in rare pediatric epilepsies. The emergence of disease-modifying and neuroprotective agents represents a paradigm shift, aiming not only to suppress seizures but also to alter the disease trajectory and improve neurodevelopmental outcomes in younger populations.
What Role Are Non-Pharmacological Modalities Playing in Refractory Epilepsy Management?
Surgical and device-based interventions are gaining clinical and commercial traction, particularly for drug-refractory epilepsy cases where pharmacotherapy alone proves insufficient. Resection surgery, including anterior temporal lobectomy and lesionectomy, continues to be a standard of care for patients with well-localized epileptogenic foci. However, advances in neuroimaging, stereoelectroencephalography (SEEG), and intraoperative neuromonitoring are expanding surgical eligibility and improving post-surgical seizure freedom rates. Laser interstitial thermal therapy (LITT), a minimally invasive technique guided by MRI, is also being adopted as a less traumatic alternative with shorter recovery periods, especially in deep-seated or eloquent cortex lesions.Neuromodulation techniques are now considered critical pillars in managing complex epilepsy profiles. Vagus nerve stimulation (VNS) has been established as an adjunctive option in both pediatric and adult populations, and newer models with closed-loop capabilities offer real-time seizure response modulation. Responsive neurostimulation (RNS), which delivers direct cortical stimulation upon early seizure detection, is demonstrating favorable outcomes in terms of seizure reduction and patient-reported quality of life. Deep brain stimulation (DBS) targeting the anterior nucleus of the thalamus has entered clinical guidelines as another option for refractory focal epilepsy. These device-based therapies are often used in conjunction with AEDs, forming integrated treatment pathways that prioritize long-term management over episodic intervention.
Where Is Patient Stratification and Digital Health Creating New Treatment Frontiers?
Patient-centric models of epilepsy management are being enabled by advances in genomics, neurodiagnostics, and digital health platforms. Genotyping and epigenetic profiling are now instrumental in identifying patients likely to respond to specific AEDs or surgical approaches, enabling stratified treatment plans. For instance, SCN1A mutation testing informs the use of sodium channel blockers in Dravet syndrome, while mTOR pathway abnormalities support the use of everolimus in tuberous sclerosis-associated epilepsy. Neuroimaging modalities such as high-resolution MRI, PET, and magnetoencephalography (MEG) are enhancing pre-surgical planning and guiding electrode placement in neuromodulation therapies.Digital tools are increasingly integrated into epilepsy care to facilitate early diagnosis, adherence monitoring, and real-time seizure tracking. Wearable seizure detectors and mobile health apps with AI-driven analytics are gaining acceptance for their role in improving patient-clinician communication and enabling.
What Is Propelling the Rapid Expansion of the Global Epilepsy Treatment Market?
The growth in the epilepsy treatment market is driven by several factors that reflect the multidimensional nature of the disease and the evolving therapeutic ecosystem. One of the primary growth catalysts is the rising prevalence of epilepsy globally, particularly in low- and middle-income countries where diagnostic infrastructure is improving and healthcare access is expanding. The increasing awareness around epilepsy as a manageable neurological condition is reducing stigma, encouraging earlier diagnosis, and driving treatment-seeking behavior. Simultaneously, policy interventions such as the WHO's Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders (IGAP) are galvanizing public health systems to prioritize epilepsy care and improve medication accessibility.Another key driver is the ongoing innovation across both pharmacological and device-based modalities. The commercial success of cannabinoid-based and genetically targeted therapies has encouraged further investment in novel mechanism drugs, while continuous improvements in neurosurgical precision and neurostimulation hardware are expanding the pool of eligible patients. Venture capital and public funding are increasingly flowing into companies developing closed-loop systems, seizure forecasting algorithms, and next-gen diagnostics, signaling long-term confidence in the sector's innovation capacity. Moreover, the establishment of epilepsy centers of excellence and cross-border collaborations in research and care delivery are accelerating knowledge dissemination and best-practice adoption.
Lastly, the demand for integrated care pathways is reshaping the epilepsy treatment ecosystem. As stakeholders from patients and caregivers to clinicians, payers, and tech developers demand more coordinated, outcome-focused solutions, the market is shifting toward bundled service models that combine diagnostics, drug therapy, device support, and digital monitoring. Reimbursement frameworks are beginning to recognize the value of holistic epilepsy care, with payers covering telemedicine consultations, neuromodulation devices, and even select digital therapeutics. With demographic shifts, policy reforms, and technological advancements converging, the epilepsy treatment market is poised for sustained expansion and deeper penetration across both developed and emerging healthcare systems.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Inpatient Diagnosis segment, which is expected to reach US$3.3 Billion by 2030 with a CAGR of a 4.8%. The Outpatient Diagnosis segment is also set to grow at 4.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.0 Billion in 2024, and China, forecasted to grow at an impressive 8.9% CAGR to reach $2.0 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Epilepsy Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Epilepsy Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Epilepsy Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Alkem Laboratories Limited, Amber Therapeutics, Amneal Pharmaceuticals LLC, and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Epilepsy Treatment market report include:
- Abbott Laboratories
- Alkem Laboratories Limited
- Amber Therapeutics
- Amneal Pharmaceuticals LLC
- Apotex Inc.
- Bausch Health Companies Inc.
- Biogen Inc.
- Boston Scientific Corporation
- Cadwell Industries, Inc.
- Cerebral Therapeutics
- Dr. Reddy's Laboratories Ltd.
- Eisai Co. Ltd.
- Eli Lilly and Company
- Epix Therapeutics
- Fisher & Paykel Healthcare Limited
- GE HealthCare Technologies Inc.
- GlaxoSmithKline plc
- Harmony Biosciences Holdings Inc.
- H. Lundbeck A/S
- Jazz Pharmaceuticals plc
- Johnson & Johnson Services Inc.
- Medtronic plc
- NeuroPace Inc.
- Natus Medical Incorporated
- Neurelis Inc.
- Novartis AG
- Otsuka America Pharmaceutical, Inc.
- Pfizer Inc.
- Sanofi
- SK Biopharmaceuticals
- Sunovion Pharmaceuticals Inc.
- Supernus Pharmaceuticals Inc.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
- UCB S.A.
- Viatris Inc.
- Zogenix Inc.
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Alkem Laboratories Limited
- Amber Therapeutics
- Amneal Pharmaceuticals LLC
- Apotex Inc.
- Bausch Health Companies Inc.
- Biogen Inc.
- Boston Scientific Corporation
- Cadwell Industries, Inc.
- Cerebral Therapeutics
- Dr. Reddy's Laboratories Ltd.
- Eisai Co. Ltd.
- Eli Lilly and Company
- Epix Therapeutics
- Fisher & Paykel Healthcare Limited
- GE HealthCare Technologies Inc.
- GlaxoSmithKline plc
- Harmony Biosciences Holdings Inc.
- H. Lundbeck A/S
- Jazz Pharmaceuticals plc
- Johnson & Johnson Services Inc.
- Medtronic plc
- NeuroPace Inc.
- Natus Medical Incorporated
- Neurelis Inc.
- Novartis AG
- Otsuka America Pharmaceutical, Inc.
- Pfizer Inc.
- Sanofi
- SK Biopharmaceuticals
- Sunovion Pharmaceuticals Inc.
- Supernus Pharmaceuticals Inc.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
- UCB S.A.
- Viatris Inc.
- Zogenix Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 483 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 7.3 Billion |
| Forecasted Market Value ( USD | $ 10.2 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |