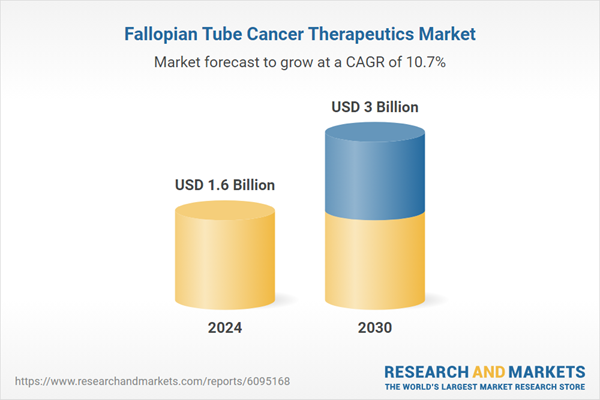
Global Fallopian Tube Cancer Therapeutics Market - Key Trends & Drivers Summarized
What Are the Latest Therapeutic Advances Reshaping Fallopian Tube Cancer Treatment?
The landscape of fallopian tube cancer therapeutics is experiencing significant innovation, driven by breakthroughs in precision medicine and molecular-targeted therapies. Among the most notable advancements are the incorporation of PARP inhibitors such as olaparib and niraparib, originally developed for ovarian cancer, but now showing promise for high-grade serous carcinomas of the fallopian tube due to overlapping genetic profiles, especially BRCA mutations. Researchers are expanding the role of next-generation sequencing (NGS) to stratify patients based on actionable genetic markers, allowing for more tailored regimens with improved efficacy and fewer systemic toxicities.Immunotherapy is also making slow yet significant strides. Although still in early-stage trials, checkpoint inhibitors targeting PD-1 and CTLA-4 have been evaluated in combination with chemotherapy or as monotherapies in recurrent and treatment-resistant cases. These innovations are gradually moving beyond academic centers into broader clinical practice, supported by updated oncology guidelines that acknowledge the unique biology and staging considerations of fallopian tube malignancies. Advances in intraoperative fluorescence-guided surgery and real-time diagnostic imaging are improving the precision of tumor resection, enhancing patient outcomes, and minimizing recurrence.
How Is the Diagnostic Ecosystem Evolving to Support Timely Intervention?
A critical challenge in the treatment of fallopian tube cancer is the difficulty in early detection due to nonspecific symptoms and anatomical obscurity. The diagnostic ecosystem, however, is being recalibrated with a growing focus on predictive biomarkers, liquid biopsies, and artificial intelligence-powered imaging tools. Liquid biopsies analyzing circulating tumor DNA (ctDNA) and exosomes are gaining traction in clinical research for their potential to detect malignancies in high-risk populations, especially those with familial cancer syndromes. When integrated with NGS, these minimally invasive diagnostics can help identify mutations in genes such as TP53, BRCA1/2, and PALB2 at earlier stages of disease progression.In parallel, diagnostic imaging is advancing through the adoption of high-resolution transvaginal ultrasonography, contrast-enhanced MRI, and AI-enhanced PET scans. These tools are proving instrumental in differentiating fallopian tube carcinoma from closely related ovarian and peritoneal malignancies. Companion diagnostics are also emerging, linking specific therapeutic agents with biomarker assays that predict patient responsiveness. As reimbursement models evolve to support precision diagnostics, more patients are expected to access these advanced tools, facilitating timely and targeted therapeutic intervention.
Which Market Dynamics and Regional Patterns Are Steering Growth?
The global fallopian tube cancer therapeutics market is shaped by a complex interplay of epidemiological shifts, regulatory reforms, and healthcare infrastructure development. North America leads the market in terms of clinical trials, regulatory approvals, and availability of advanced therapeutics, largely due to established cancer research centers and favorable insurance coverage. Europe follows closely, with countries like Germany and the UK investing in population-wide screening initiatives and genomic profiling programs. In Asia-Pacific, the market is still maturing but shows substantial potential owing to increasing awareness, rising cancer incidence, and government-led investments in oncology diagnostics and treatment access.Biopharmaceutical partnerships and academic-industry collaborations are fueling innovation pipelines. Several multinational drugmakers are entering licensing deals with biotech firms focused on rare gynecological cancers, aiming to expand their oncology portfolios. Pricing pressures remain a constraint in lower-income economies, where access to targeted therapies is often limited. However, the introduction of biosimilars and tiered pricing models is expected to alleviate some cost barriers. The presence of patient advocacy groups and cancer foundations is further influencing treatment guidelines and access through funding, awareness campaigns, and lobbying for regulatory fast-tracking of promising therapies.
What Forces Are Driving the Expansion of the Therapeutics Market?
The growth in the fallopian tube cancer therapeutics market is driven by several factors that converge across clinical, technological, and economic domains. One of the key drivers is the growing inclusion of fallopian tube cancer in gynecologic oncology research and trial designs. Historically grouped with ovarian cancer, fallopian tube carcinoma is now being recognized as a distinct pathological entity, leading to focused R&D efforts, expanded patient registries, and refined staging criteria. This classification shift is helping pharmaceutical companies and research institutions justify investment in dedicated therapeutic development.Another catalyst is the broadening application of personalized medicine and biomarker-led therapy. The routine screening for BRCA mutations and homologous recombination deficiency (HRD) is enabling stratified treatment approaches, reducing trial-and-error prescriptions, and improving progression-free survival rates. Furthermore, the continued rollout of global oncology clinical trials is expanding treatment options across geographies, accelerating the regulatory approval process for targeted agents.
Health system modernization - particularly in emerging economies - is playing a supporting role. As more hospitals are equipped with genomic testing capabilities and digital health tools, the penetration of advanced therapeutics is expected to rise. Educational campaigns led by oncology associations and global health bodies are improving patient awareness and driving early-stage diagnosis, while AI-powered clinical decision support tools are guiding oncologists toward optimal treatment algorithms. These ecosystem-wide improvements are setting the stage for sustained growth in the fallopian tube cancer therapeutics market, with promising long-term implications for both patient outcomes and commercial viability.
Report Scope
The report analyzes the Fallopian Tube Cancer Therapeutics market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Targeted Therapy, Chemotherapy); End-Use (Hospitals End-Use, Clinics & Ambulatory Surgery Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Targeted Therapy segment, which is expected to reach US$2.2 Billion by 2030 with a CAGR of a 11.7%. The Chemotherapy segment is also set to grow at 8.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $429.1 Million in 2024, and China, forecasted to grow at an impressive 9.7% CAGR to reach $461.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Fallopian Tube Cancer Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Fallopian Tube Cancer Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Fallopian Tube Cancer Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABB Ltd., Amphenol Corporation, Analog Devices, Inc., Balluff GmbH, Banner Engineering Corp and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Fallopian Tube Cancer Therapeutics market report include:
- AbbVie Inc.
- Advenchen Laboratories LLC
- AiVita Biomedical Inc.
- Alkermes plc
- Astellas Pharma Inc.
- Astex Pharmaceuticals Inc.
- Celsion Corporation
- Compugen Ltd.
- CSPC ZhongQi Pharmaceutical Technology Co., Ltd.
- Eli Lilly and Company
- Exelixis Inc.
- Genentech Inc.
- Genmab A/S
- ImmunoGen Inc.
- Janssen Research & Development (Johnson & Johnson)
- Merck & Co., Inc.
- OncoQuest Pharmaceuticals Inc.
- Pfizer Inc.
- Regeneron Pharmaceuticals Inc.
- Syndax Pharmaceuticals Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Advenchen Laboratories LLC
- AiVita Biomedical Inc.
- Alkermes plc
- Astellas Pharma Inc.
- Astex Pharmaceuticals Inc.
- Celsion Corporation
- Compugen Ltd.
- CSPC ZhongQi Pharmaceutical Technology Co., Ltd.
- Eli Lilly and Company
- Exelixis Inc.
- Genentech Inc.
- Genmab A/S
- ImmunoGen Inc.
- Janssen Research & Development (Johnson & Johnson)
- Merck & Co., Inc.
- OncoQuest Pharmaceuticals Inc.
- Pfizer Inc.
- Regeneron Pharmaceuticals Inc.
- Syndax Pharmaceuticals Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 139 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 1.6 Billion |
| Forecasted Market Value ( USD | $ 3 Billion |
| Compound Annual Growth Rate | 10.7% |
| Regions Covered | Global |