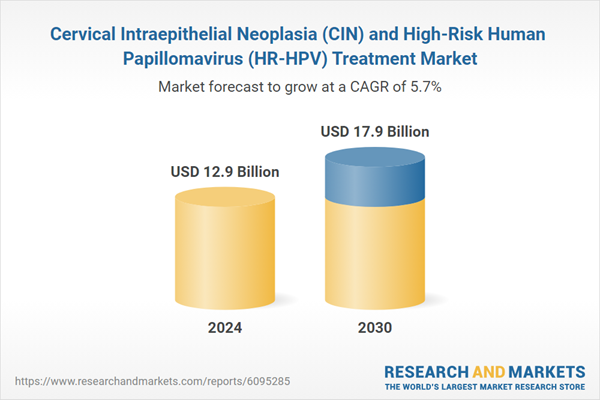
Global Cervical Intraepithelial Neoplasia (CIN) and High-Risk Human Papillomavirus (HR-HPV) Treatment Market - Key Trends & Drivers Summarized
Why Is CIN and HR-HPV Treatment Drawing Greater Global Focus?
The market for treating cervical intraepithelial neoplasia (CIN) and high-risk human papillomavirus (HR-HPV) is experiencing significant growth due to rising global awareness around cervical cancer prevention and the increasing implementation of screening and vaccination programs. CIN represents the precancerous changes in the cervix caused primarily by persistent infection with high-risk strains of HPV, most notably types 16 and 18. As one of the most preventable forms of cancer, cervical cancer has become a priority for public health systems, particularly in low- and middle-income countries where mortality rates remain high due to lack of access to early detection and treatment.The World Health Organization's global strategy to eliminate cervical cancer as a public health problem is catalyzing investments in early screening technologies, such as Pap smears and HPV DNA testing, and in effective therapeutic interventions. As more women are diagnosed with CIN grades I-III, the demand for timely and minimally invasive treatment options - ranging from ablative and excisional procedures to emerging pharmacological therapies - is surging. This market momentum is also supported by rising government and NGO funding for reproductive health initiatives, which prioritize cervical cancer prevention and HPV management across underserved populations.
How Are Diagnostic Innovations Improving Treatment Outcomes?
Technological advancements in diagnostics are significantly impacting the CIN and HR-HPV treatment landscape by enabling earlier, more accurate, and less invasive detection. High-sensitivity molecular testing methods, including HPV genotyping and liquid-based cytology, are becoming standard tools for identifying women at highest risk of disease progression. These methods offer superior detection rates over traditional cytology and are particularly effective in identifying asymptomatic or latent infections that might otherwise be missed. Such early detection increases the chances of successful treatment and significantly reduces the burden of invasive disease.Point-of-care testing (POCT) is also emerging as a game-changer in remote and resource-constrained settings. Rapid HPV tests that provide results within hours are allowing for a “screen-and-treat” approach, reducing patient drop-off between diagnosis and intervention. In parallel, AI-powered digital colposcopy and automated cytology platforms are improving diagnostic accuracy while reducing reliance on specialist interpretation. These innovations are narrowing the diagnostic gap between high-income and developing regions, enabling broader population coverage and more equitable treatment access, thereby expanding the global footprint of the CIN and HR-HPV treatment market.
Why Are Treatment Modalities Diversifying Beyond Surgery?
Treatment of CIN and HR-HPV is no longer confined to conventional surgical or ablative procedures. There is a clear trend toward less invasive, patient-friendly modalities that preserve fertility and minimize post-treatment complications. Thermal ablation, cryotherapy, and laser treatments are gaining ground as frontline options for low- and mid-grade lesions (CIN1 and CIN2), especially in settings where surgical infrastructure is limited. Loop electrosurgical excision procedure (LEEP) and cold knife conization remain standards for high-grade lesions, but these are increasingly supplemented or even replaced by pharmacological therapies in clinical trials.Emerging topical and systemic immunotherapeutic agents aim to clear HPV infections and reverse precancerous changes without the need for invasive procedures. Therapeutic vaccines targeting HPV oncoproteins, and gene-editing approaches using CRISPR-based systems, are under development as part of next-generation treatment strategies. The pharmaceutical pipeline is robust, with numerous candidates seeking to address persistent infection and prevent progression to invasive cervical cancer. These evolving treatment paradigms are expanding clinical options, enabling more personalized, less invasive care that meets the diverse needs of women across age groups and geographies.
What Are the Core Drivers Catalyzing Market Growth?
The growth in the cervical intraepithelial neoplasia (CIN) and high-risk HPV treatment market is driven by several factors. The global increase in HPV infections - partly due to early onset of sexual activity and limited vaccination coverage in many regions - is raising the prevalence of CIN and associated complications. Improvements in diagnostic accuracy through HPV genotyping, AI-enhanced colposcopy, and rapid testing are enabling earlier detection and intervention. Public health efforts, including national screening programs and international campaigns to eliminate cervical cancer, are pushing treatment demand to new levels. Additionally, the diversification of treatment options - from thermal ablation and LEEP to novel pharmacological and immunological approaches - is making management more accessible and less invasive. Rising healthcare investments in women's reproductive health, especially in developing nations, are further driving adoption. Combined, these technology, epidemiology, policy, and clinical innovation trends are fueling steady and widespread growth in the global CIN and HR-HPV treatment market.Report Scope
The report analyzes the Cervical Intraepithelial Neoplasia (CIN) and High-Risk Human Papillomavirus (HR-HPV) Treatment market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Offering (Kits & Reagents Offering, Instruments Offering, Services Offering); Disease Type (Cervical Intraepithelial Neoplasia 1, Cervical Intraepithelial Neoplasia 2, Cervical Intraepithelial Neoplasia 3); Strain Type (HPV 16, HPV 18).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Kits & Reagents segment, which is expected to reach US$10.4 Billion by 2030 with a CAGR of a 6.9%. The Instruments segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.5 Billion in 2024, and China, forecasted to grow at an impressive 9.2% CAGR to reach $3.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Cervical Intraepithelial Neoplasia (CIN) and High-Risk Human Papillomavirus (HR-HPV) Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Cervical Intraepithelial Neoplasia (CIN) and High-Risk Human Papillomavirus (HR-HPV) Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Cervical Intraepithelial Neoplasia (CIN) and High-Risk Human Papillomavirus (HR-HPV) Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alrosa PJSC, Anglo American plc (De Beers Group), Arctic Star Exploration Corp., Blue Nile Inc., Botswana Diamonds Plc and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Cervical Intraepithelial Neoplasia (CIN) and High-Risk Human Papillomavirus (HR-HPV) Treatment market report include:
- Advaxis, Inc.
- Antiva Biosciences, Inc.
- Barinthus Biotherapeutics plc
- Bioleaders Corp.
- Blue Sky Immunotherapies GmbH
- CEL-SCI Corporation
- Frantz Viral Therapeutics LLC
- Genexine, Inc.
- GlaxoSmithKline plc
- Inovio Pharmaceuticals, Inc.
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Novan, Inc.
- Nykode Therapeutics ASA
- Papivax LLC
- PDS Biotechnology Corporation
- Pfizer Inc.
- Procare Health Iberia S.L.
- Transgene S.A.
- Vaxart, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Advaxis, Inc.
- Antiva Biosciences, Inc.
- Barinthus Biotherapeutics plc
- Bioleaders Corp.
- Blue Sky Immunotherapies GmbH
- CEL-SCI Corporation
- Frantz Viral Therapeutics LLC
- Genexine, Inc.
- GlaxoSmithKline plc
- Inovio Pharmaceuticals, Inc.
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Novan, Inc.
- Nykode Therapeutics ASA
- Papivax LLC
- PDS Biotechnology Corporation
- Pfizer Inc.
- Procare Health Iberia S.L.
- Transgene S.A.
- Vaxart, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 364 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 12.9 Billion |
| Forecasted Market Value ( USD | $ 17.9 Billion |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |