Global Clinical Trial Management Software (CTMS) Market - Key Trends & Drivers Summarized
The global market for Clinical Trial Management Software (CTMS) is witnessing a rapid evolution as the biopharmaceutical industry embraces digitization to streamline and scale complex clinical operations. CTMS platforms are no longer viewed as optional administrative tools but as strategic systems central to managing the lifecycle of clinical trials from protocol planning to site performance monitoring. As clinical trials become more globalized and.Modern CTMS platforms are being designed to integrate seamlessly with electronic data capture (EDC), laboratory information systems (LIS), and patient engagement tools. These integrations enable sponsors and CROs to centralize trial oversight, reduce data silos, and automate performance reporting. The shift toward unified platforms also allows stakeholders to gain visibility into key trial metrics such as enrollment rates, adverse events, and budget tracking, all in real time. As a result, CTMS tools are becoming essential not just for operations teams, but also for strategic decision-makers seeking to accelerate time-to-market while maintaining quality and compliance.
What Innovations Are Driving the Digital Shift in Trial Management?
The clinical trial ecosystem is embracing next-generation technologies to transform how trials are managed. Artificial intelligence and machine learning are being embedded into CTMS platforms to enable predictive analytics, such as forecasting site performance or identifying at-risk patient cohorts. Similarly, robotic process automation (RPA) is being deployed to handle repetitive administrative tasks like site payments, visit scheduling, and protocol deviation alerts. These advances are freeing up human resources to focus on value-added activities, while also minimizing trial delays and improving data accuracy.Cloud-based deployment models are becoming the norm, offering scalability, reduced IT overheads, and faster implementation timelines. In tandem, user-friendly dashboards and mobile-compatible interfaces are improving system adoption among clinical operations teams. The growing popularity of hybrid and decentralized trials is pushing vendors to develop modules that support remote monitoring, virtual investigator meetings, and patient-reported outcomes all within the CTMS environment. Furthermore, blockchain technology is gaining interest for ensuring tamper-proof audit trails and secure contract management within CTMS platforms, especially in multi-party trial ecosystems.
How Are End-Users Shaping Software Functionality and Deployment Models?
End-user demands are increasingly defining how CTMS solutions are built, configured, and deployed. Large pharmaceutical companies require enterprise-grade platforms with customizable modules, multi-region support, and integration capabilities that align with their broader IT architecture. These users often demand compliance with global regulatory frameworks such as FDA 21 CFR Part 11 and EMA GCP guidelines, necessitating robust access control, electronic signatures, and audit functionality.Meanwhile, small to mid-sized biotech firms and academic research organizations are gravitating toward SaaS-based CTMS platforms that offer plug-and-play capabilities with minimal setup time. These users prioritize affordability, flexibility, and vendor support often preferring modular systems that allow gradual scaling as research pipelines expand. Contract research organizations (CROs), on the other hand, seek systems optimized for multi-sponsor workflows, quick study startups, and configurable templates that reduce trial setup timelines. As more public-private collaborations and government-sponsored trials emerge, demand is rising for multi-tenant CTMS systems that support diverse stakeholder access and data compartmentalization.
What Forces Are Accelerating the Growth of the CTMS Market?
The growth in the clinical trial management software market is driven by several factors closely tied to operational innovation, regulatory complexity, and evolving research models. One of the core drivers is the increase in clinical trial volume across oncology, rare diseases, and vaccine development, all of which necessitate sophisticated trial oversight and documentation. The growing reliance on real-time data analytics for trial optimization is also fueling adoption of advanced CTMS tools that provide end-to-end visibility and actionable insights.Regulatory expectations for electronic documentation, audit readiness, and data traceability are another significant force prompting organizations to transition from legacy systems or spreadsheets to compliant CTMS platforms. Additionally, the rise of virtual and decentralized trials has created new requirements for remote monitoring, centralized reporting, and participant tracking capabilities well-supported by modern CTMS systems. Strategic investments from software vendors in AI, data interoperability, and user-centric design are further boosting the attractiveness of CTMS solutions. As digital transformation becomes a top priority in life sciences, CTMS is evolving into a critical enabler of efficiency, transparency, and speed in the clinical research process.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Enterprise Deployment segment, which is expected to reach US$2.3 Billion by 2030 with a CAGR of a 16.6%. The On-Site Deployment segment is also set to grow at 10.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $358.2 Million in 2024, and China, forecasted to grow at an impressive 20.2% CAGR to reach $667.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Clinical Trial Management Software (CTMS) Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Clinical Trial Management Software (CTMS) Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Clinical Trial Management Software (CTMS) Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Advarra, ArisGlobal, Bio-Optronics, BioClinica, and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Clinical Trial Management Software (CTMS) market report include:
- Advarra
- ArisGlobal
- Bio-Optronics
- BioClinica
- Calyx (formerly Parexel Informatics)
- Clario
- CluePoints
- Cytel
- DATATRAK International, Inc.
- DSG (Document Solutions Group)
- eClinical Solutions
- eClinForce
- Ennov
- Forte Research Systems
- Genedata
- IBM
- ICON plc
- Medidata Solutions
- MedNet Solutions
- Oracle Corporation
- Parexel International
- RealTime Software Solutions
- Veeva Systems
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Advarra
- ArisGlobal
- Bio-Optronics
- BioClinica
- Calyx (formerly Parexel Informatics)
- Clario
- CluePoints
- Cytel
- DATATRAK International, Inc.
- DSG (Document Solutions Group)
- eClinical Solutions
- eClinForce
- Ennov
- Forte Research Systems
- Genedata
- IBM
- ICON plc
- Medidata Solutions
- MedNet Solutions
- Oracle Corporation
- Parexel International
- RealTime Software Solutions
- Veeva Systems
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 279 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
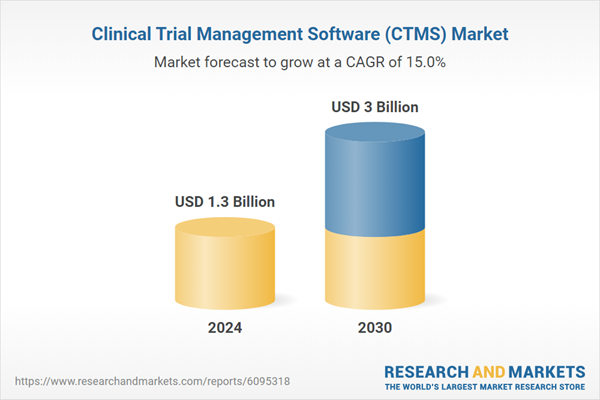
| Estimated Market Value ( USD | $ 1.3 Billion |
| Forecasted Market Value ( USD | $ 3 Billion |
| Compound Annual Growth Rate | 15.0% |
| Regions Covered | Global |