Global Bronchial Thermoplasty Catheters Market - Key Trends & Drivers Summarized
Why Are Bronchial Thermoplasty Catheters Gaining Traction as a Minimally Invasive Solution for Severe Asthma Management?
Bronchial thermoplasty catheters are emerging as a specialized intervention for patients with severe, persistent asthma that remains uncontrolled despite maximal inhaled therapy. These devices deliver controlled thermal energy to the airway wall to reduce excess smooth muscle tissue - resulting in decreased bronchoconstriction, improved airflow, and reduced asthma exacerbations. As global asthma prevalence continues to rise and traditional pharmacologic options prove inadequate for a subset of patients, bronchial thermoplasty is gaining clinical traction as a long-term, procedure-based therapy for disease control.This non-pharmacologic, minimally invasive treatment aligns with the shift toward personalized, intervention-based chronic respiratory care. Supported by clinical evidence demonstrating reductions in emergency room visits, hospitalization rates, and steroid dependency, bronchial thermoplasty catheters offer a therapeutic alternative for high-risk patients who are not candidates for or do not respond adequately to biologics. The procedure's one-time intervention model also appeals to payers and health systems aiming to reduce long-term disease management costs.
How Are Device Advancements and Procedural Standardization Improving Adoption of Bronchial Thermoplasty?
Advancements in catheter design, energy control algorithms, and bronchoscopic delivery systems are enhancing procedural safety, precision, and operator confidence. Modern bronchial thermoplasty catheters feature flexible, tapered designs with electrode arrays that conform to airway anatomy and deliver uniform radiofrequency energy while minimizing mucosal trauma. Real-time temperature monitoring, safety interlocks, and dose-limiting protocols further reduce complications and support reproducible outcomes across treatment sessions.Training programs, procedural checklists, and standardized patient selection criteria are streamlining clinical workflows and improving treatment consistency. Integration with advanced bronchoscopes and imaging systems is enabling improved visualization, navigation, and lesion targeting. These improvements are making the procedure more accessible to interventional pulmonologists and supporting wider clinical acceptance in academic, hospital, and specialty care environments.
Where Is Demand for Bronchial Thermoplasty Catheters Increasing and Which Patient Populations Are Driving Utilization?
North America remains the leading market for bronchial thermoplasty, driven by early FDA approval, reimbursement coverage in select U.S. regions, and strong specialist infrastructure. Europe follows with steady adoption in countries such as Germany, France, and the U.K., where healthcare systems are integrating bronchial thermoplasty into severe asthma management guidelines. Asia-Pacific is seeing emerging interest, particularly in Japan, South Korea, and Australia, where clinical awareness and asthma prevalence are high.Primary patient populations include adults with severe, refractory asthma not controlled by high-dose inhaled corticosteroids and long-acting beta-agonists, and who have frequent exacerbations or corticosteroid dependence. Bronchial thermoplasty is also considered for patients with contraindications to long-term biologic therapy or poor adherence to daily inhaler regimens. Payers and clinicians are increasingly targeting this intervention toward patients with high healthcare utilization or poor quality-of-life metrics - seeking both symptom control and cost mitigation.
What Is Fueling the Global Growth of the Bronchial Thermoplasty Catheters Market?
The global bronchial thermoplasty catheter market is being driven by the rising clinical burden of severe asthma, growing limitations of conventional therapies, and increasing healthcare interest in interventional, cost-effective disease management approaches. As asthma-related hospitalizations and systemic steroid use remain significant public health concerns, bronchial thermoplasty offers a one-time procedural solution with demonstrated long-term benefits.Supportive clinical trial data, growing physician familiarity, and expanding insurance coverage are reinforcing adoption, particularly in specialized pulmonary centers. Ongoing technological refinements, combined with patient education and multidisciplinary asthma management programs, are further improving outcomes and acceptance. As global respiratory care pivots toward precision and procedure-based intervention, a defining question shapes the future of this niche segment: Can bronchial thermoplasty catheters continue to demonstrate durable efficacy, procedural scalability, and payer-aligned value - while establishing a stable foothold in the broader therapeutic landscape for severe asthma?
Report Scope
The report analyzes the Bronchial Thermoplasty Catheters market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: End-User (Hospitals, Ambulatory Surgery Centers).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Hospitals End-Use segment, which is expected to reach US$2.9 Billion by 2030 with a CAGR of a 2.6%. The Ambulatory Surgery Centers End-Use segment is also set to grow at 4.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1 Billion in 2024, and China, forecasted to grow at an impressive 5.9% CAGR to reach $904.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Bronchial Thermoplasty Catheters Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Bronchial Thermoplasty Catheters Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Bronchial Thermoplasty Catheters Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABB Robotics, Advanced Construction Robotics, Apis Cor, Asmbld, Autonomous Solutions Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Bronchial Thermoplasty Catheters market report include:
- Abbott Laboratories
- AngioDynamics, Inc.
- Becton, Dickinson & Co.
- Boehringer Ingelheim
- Boston Scientific Corp.
- C. R. Bard, Inc.
- CONMED Corporation
- Cook Medical LLC
- Johnson & Johnson
- Karl Storz GmbH & Co. KG
- Medtronic plc
- Merit Medical Systems
- Neomedic International
- Olympus Corporation
- Penumbra, Inc.
- Pulmonx Corporation
- Smith & Nephew plc
- Stryker Corporation
- Teleflex Incorporated
- Terumo Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- AngioDynamics, Inc.
- Becton, Dickinson & Co.
- Boehringer Ingelheim
- Boston Scientific Corp.
- C. R. Bard, Inc.
- CONMED Corporation
- Cook Medical LLC
- Johnson & Johnson
- Karl Storz GmbH & Co. KG
- Medtronic plc
- Merit Medical Systems
- Neomedic International
- Olympus Corporation
- Penumbra, Inc.
- Pulmonx Corporation
- Smith & Nephew plc
- Stryker Corporation
- Teleflex Incorporated
- Terumo Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 170 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
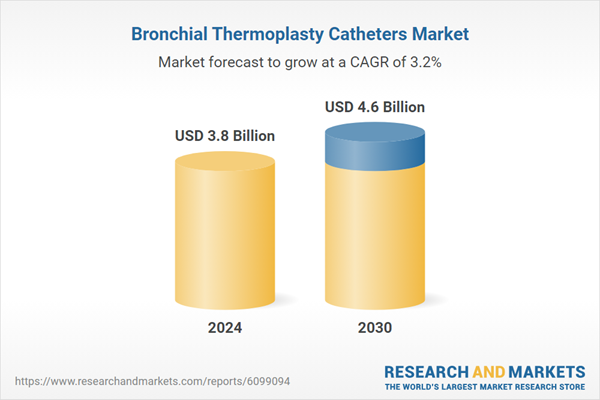
| Estimated Market Value ( USD | $ 3.8 Billion |
| Forecasted Market Value ( USD | $ 4.6 Billion |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | Global |