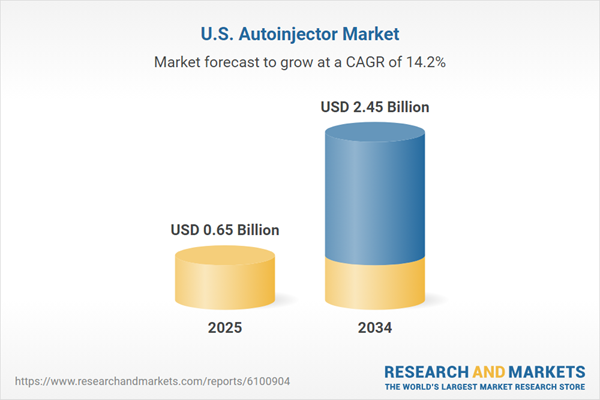
United States Autoinjector Market Overview
Autoinjector devices are prefilled, self-administering drug delivery systems designed for rapid, accurate, and convenient injection without professional assistance. The market for autoinjectors in the United States is experiencing significant growth, driven by rising chronic disease prevalence, increasing self-medication trends, and technological advancements enhancing patient compliance and safety.United States Autoinjector Market Growth Drivers
Next-Generation Testing Technology Set to Augment Market Demand
Integrating advanced testing systems will enhance the reliability and quality assurance of autoinjector devices. For instance, in June 2024, Instron launched its next-generation autoinjector testing system, enabling comprehensive functionality testing on a single platform. By streamlining validation and production transfer processes, this innovation supports efficiency and product safety, ultimately accelerating market growth.United States Autoinjector Market Trends
Some of the notable trends in the market include expanding regulatory approvals for injectable therapies and increasing government initiatives focused on emergency preparedness and access to critical self-injection treatments.Regulatory Advancements is Poised to Accelerate Market Growth
Expanding regulatory approvals for treatments represent a significant trend in the autoinjector devices market. In August 2024, the U.S. FDA approved Zurnai, the first nalmefene hydrochloride auto-injector for emergency opioid overdose treatment. This milestone, in alignment with the FDA’s overdose prevention framework, is anticipated to foster innovation and accelerate market growth.Rising Government Initiatives in Emergency Preparedness is Likely to Elevate United States Autoinjector Market Value
A growing trend in the market is the increased investment in emergency preparedness and public health response. In April 2025, Barda’s funding to Kaléo for developing 2-PAM autoinjectors highlighted the government’s commitment to enhancing rapid-response capabilities. Such initiatives are expected to drive market growth by advancing autoinjector innovation and expanding strategic stockpiles.
United States Autoinjector Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Type
- Disposable
- Reusable
- Empty
- Prefilled
Market Breakup by Volume
- Up to 3ml
- Above 3ml
Market Breakup by Technology
- Automatic
- Manual
Market Breakup by Route of Administration
- Subcutaneous
- Intramuscular
Market Breakup by Application
- Autoimmune Disorders
- Rheumatoid Arthritis
- Diabetes
- Multiple Sclerosis
- Anaphylaxis
- Others
Market Breakup by End User
- Hospitals & Clinics
- Homecare Settings
- Ambulatory Surgical Centers
- Others
United States Autoinjector Market Share
Segmentation Based on Application to Witness Substantial Growth
Based on the type, the market is segmented into disposable and reusable. The disposable segment is anticipated to have a substantial share of the market, driven by increasing demand for hygienic and single-use medical products across healthcare settings. Enhanced infection control protocols, cost-effectiveness in bulk procurement, and minimal maintenance requirements contribute to its widespread adoption. Additionally, the growing preference in outpatient and homecare environments further strengthens the segment's projected market dominance.United States Autoinjector Market Analysis by Region
The autoinjector devices market in the United States demonstrates strong regional performance, with leading growth observed in the Mideast and Southeast regions. These areas benefit from advanced healthcare infrastructure, high chronic disease prevalence, and increasing patient awareness. Additionally, the Southwest regions contribute significantly, driven by rising demand for self-administration solutions and supportive healthcare policies.Leading Players in the United States Autoinjector Market
The key features of the market report comprise patent analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Eli Lilly and Company
Eli Lilly and Company, founded in 1876 and headquartered in Indianapolis, Indiana, plays a pivotal role in the market. In August 2024, the company expanded access to Zepbound (tirzepatide) by introducing single-dose vials and autoinjector pens for adults with obesity. This launch enhances treatment accessibility and supports patients with self-administered, subcutaneous weight management solutions.Teva Pharmaceuticals: Teva Pharmaceuticals plays a significant role in the autoinjector market. It was established in 1901 and is headquartered in Tel Aviv, Israel. The company offers Epinephrine injection, USP (auto-injector), a generic equivalent to EpiPen and EpiPen Jr. These pre-filled, single-dose autoinjectors are used in the emergency treatment of allergic reactions, highlighting Teva’s commitment to accessible, life-saving therapies.Ypsomed
Ypsomed, headquartered in Burgdorf, Switzerland, was established in 1984 and is actively contributing to the autoinjector devices market. The company’s innovative product, YpsoMate On, is a pre-filled autoinjector featuring integrated connectivity that logs injections automatically and enhances therapy adherence. Recognized with the Pharmapack 2022 Innovation Award, it supports digital therapy management with a sustainable, user-friendly design.SHL Medical
SHL Medical, established in 1989, is headquartered in Switzerland. The company specializes in the development, manufacturing, and assembly of drug delivery devices, including autoinjectors. In 2022, SHL Medical expanded into the U.S. market with a new facility in North Charleston, South Carolina. Their product lineup includes the Molly autoinjectors, designed for self-injection systems, crucial for treating chronic diseases. This move enhances their ability to meet growing demand in the U.S. autoinjector devices market.Other key players in the market include Pfizer, Inc., Amgen, Sanofi, Mylan N.V., and Biogen.
Key Questions Answered in the United States Autoinjector Market Report
- What was the United States autoinjector market value in 2024?
- What is the United States autoinjector market forecast outlook for 2025-2034?
- What is the market segmentation based on type?
- What is the market segmentation based on volume?
- What is the market segmentation based on technology?
- What is the market breakup based on the route of administration?
- How is the market segmented based on application?
- How is the market divided based on the end user?
- What are the major factors aiding the United States autoinjector market demand?
- How has the market performed so far, and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major United States autoinjector market trends?
- Which type is expected to dominate the market segment?
- Which volume is expected to dominate the market segment?
- Which technology is expected to dominate the market segment?
- Which route of administration is projected to lead the market segment?
- Which application is anticipated to drive the market segment?
- Which end user is likely to dominate the market segment?
- Who are the key players involved in the United States autoinjector market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Eli Lilly and Company
- Ypsomed
- SHL Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 0.65 Billion |
| Forecasted Market Value ( USD | $ 2.45 Billion |
| Compound Annual Growth Rate | 14.2% |
| Regions Covered | United States |
| No. of Companies Mentioned | 3 |