Glioma Market Overview
Gliomas are tumors that begin in the brain or spinal cord, consisting of cells that look like normal glial cells providing aid to nerve activity. As glioma grows, it forms a mass that can cause compression on brain or spinal cord tissue, leading to symptoms specific to the affected area. Different types of gliomas are present, with some growing slowly and not considered cancerous, and others, known as malignant gliomas, spreading quickly to neighboring healthy tissue. There are four different grades of differentiation that gliomas can exhibit. Grade I gliomas are the most differentiated and least malignant, whereas Grade IV tumors are the least differentiated and most malignant. The type of glioma determines the seriousness of the condition and the appropriate course of treatment.Gliomas may progress to brain tumors, penetrating and harming healthy tissue. In general, tumors called gliomas impact glial cells that support nerves and can cause issues as they expand and impact nearby tissues. The process of diagnosing glioma includes neurological tests, angiograms, MRI scans, CT scans, and surgical biopsies. Seizures, headaches, mood changes, and difficulty walking lead the patient to see a general practitioner, who then recommends a visit to a neuro-oncologist.
Glioma Market Growth Drivers
Impact of Increasing Glioma Prevalence on Market Dynamics
The increasing prevalence of glioma is driving demand for advanced glioma treatments. Factors such as aging populations, genetic mutations, and environmental risks like radiation contribute to the rising glioma cases. Approximately 80,000 new instances of primary brain tumors are diagnosed annually in the United States, with around 25% being gliomas. Around 12,000 cases of glioblastomas are diagnosed annually. Advanced imaging technologies and early detection efforts have improved identification rates, while ongoing research initiatives enhance understanding and reporting of glioma incidences. To address this growing concern, healthcare providers are focusing on early detection through enhanced screening and molecular diagnostics, while pharmaceutical companies and medical device manufacturers are developing targeted therapies and improving imaging technologies. Collaboration among advocacy groups, healthcare organizations, and research institutions aims to drive continuous advancements in the glioma market.Advancements in Treatment to Address Rising Glioma Market Demand
Advancements in glioma treatment like minimally invasive surgeries and intraoperative imaging tools, lower surgical dangers and enhanced accuracy are rising in the market. Focused treatments such as molecularly targeted drugs and immunotherapies provide tailored care depending on genetic and molecular tumor characteristics. Advancements in radiation therapy, such as stereotactic radiosurgery and proton therapy, have enhanced treatment by precisely targeting tumors with high doses of radiation. Furthermore, improvements in chemotherapy have increased effectiveness while reducing overall side effects.Glioma Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Advancements in Diagnostic Technologies
MRI, perfusion imaging, and spectroscopy offer in-depth data for precise tumor diagnosis and visualization. Molecular diagnostics help identify genetic mutations and biomarkers, which inform personalized treatment plans. Advancements in biopsies decrease invasiveness and enhance precision in identifying tumor traits. Artificial intelligence in diagnostic processes improves precision and effectiveness by forecasting tumor advancement and aiding in clinical decision-making. In general, these technologies represent a revolutionary era in glioma diagnostics, focusing on early identification, tailored therapy, and better patient results.Rising Emphasis on Research
Advancements in glioma research are expected to improve the understanding of the disease's biology, leading to new treatment targets and strategies. Pharmaceutical companies are investing in developing specialized drugs for glioma patients. Research also enhances diagnostic capabilities, allowing for earlier detection and personalized treatment plans. Collaboration among academia, industry, and healthcare providers promotes knowledge and enables sharing of best practices in managing glioma.Awareness and Education
Raising knowledge and promoting education on gliomas is crucial for generating interest in treatments. Educational initiatives aimed at healthcare professionals and the public can lead to early detection and intervention. Patients who are knowledgeable tend to seek specialized care and take part in treatment decisions more often.Government and Non-Governmental Initiatives
Government organizations are providing funding for glioma research to enhance treatment, diagnostics, and patient care, leading to improved accessibility to public healthcare and better outcomes. The partnership among governments, NGOs, and pharmaceutical corporations speeds up clinical trials and authorizations, broadening treatment choices and enhancing worldwide patient care.Glioma Market Segmentation
The EMR’s report titled “Glioma Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Treatment Type
- Chemotherapy
- Radiation Therapy
- Targeted Drug Therapy
- Others
Market Breakup by Type
- Low Grade
- High Grade
Market Breakup by End User
- Hospital/Clinics
- Ambulatory Surgical Centers
- Cancer Centers
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Glioma Market Share
Market Segmentation Based on Treatment Type to Witness Growth
The market segmentation based on treatment type includes chemotherapy, radiation therapy, targeted drug therapy and others. Chemotherapy uses potent drugs to kill cancer cells or prevent their growth. It is given orally or intravenously, circulating throughout the body. It is expected to hold a significant market share in the forecast period. Radiation therapy utilizes high-energy rays to kill cancer cells in a targeted area. It is frequently used in glioma treatment to shrink difficult-to-remove tumors or treat residual cells post-surgery, focusing on specific tumor locations.Glioma Market Analysis by Region
Based on region, the market report covers the United States, EU-4 (Germany, France, Italy, and Spain), the United Kingdom, Japan, and India. The United States is expected to dominate the glioma market due to its advanced healthcare services and high prevalence of glioma. Big pharmaceutical companies and robust regulations support market expansion in the region. Increased awareness is improving diagnostic technologies, and a growing elderly population is fueling the need for glioma treatments, resulting in market demand.The EU-4 and United Kingdom market is also experiencing robust growth because of well-developed healthcare systems and research programs that push forward innovations in treating glioma. Partnerships between academic institutions and pharmaceutical companies are resulting in the development of novel treatments. The market in Japan and India is also experiencing significant growth. The glioma market in Japan is boosted by cutting-edge healthcare technologies and personalized medicine.
Leading Players in the Glioma Market
The key features of the market report include patent analysis, clinical trials analysis, grant analysis, funding, and investment analysis as well as strategic initiatives by the leading players. The major companies in the market are as follows:Denovo Biopharma LLC
Denovo Biopharma, established in 2012, specializes in creating precision medicines driven by biomarkers through a technology platform that enables biomarker discovery and assists in drug development. Their fundamental technology allows for the identification of new genomic biomarkers in different areas of therapy. In July 2023, the FDA approved a Phase 2 trial of DB107 for the treatment of high-grade glioma.AIVITA Biomedical, Inc
AIVITA Biomedical, established in 2016, specializes in creating personalized vaccines for pathogens and tumor-initiating cells. Their system applies stem cell knowledge for the secure and cost-effective production of market goods and treatments.Northwest Biotherapeutics, Inc
Northwest Biotherapeutics, Inc., a pharmaceutical company established in 1996 in Maryland, USA, is dedicated to creating tailored immunotherapy products for treating cancer. Their flagship product, DCVax®-L, is focused on glioblastoma, known as the most aggressive type of brain cancer. The company has finished Phase III trials, released findings, and requested UK commercial approval. DCVax®-Direct has been created for solid tumors as well.AstraZeneca
AstraZeneca, established in 1999, is a pharmaceutical company based in Britain and Sweden. The company is based in Cambridge, England, and offers a range of products for conditions such as oncology, cardiovascular, and respiratory illnesses. In April 2024, the outcomes of AZD1390 were encouraging for individuals with Glioblastoma.Other players in the market include Bayer AG, Bristol-Myers Squibb Company, Chimerix, CNS Pharmaceuticals, Inc., ERC SA., Immunomic Therapeutics, Inc., Laminar Pharmaceuticals, S.A., Medicenna Therapeutics, MimiVax, Inc., and VBL Therapeutics.
Key Questions Answered in the Glioma Market Report
- What was the glioma market value in 2024?
- What is the glioma market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on treatment type?
- What is market segmentation based on type?
- What are the major end users in the market?
- What are the major factors aiding the glioma market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the major drivers, opportunities, and restraints in the market?
- What are the major trends influencing the market?
- Which treatment type will dominate the market?
- Which regional market is expected to dominate the market share in the forecast period?
- Which country is likely to experience elevated growth during the forecast period?
- Who are the key players involved in the glioma market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Denovo Biopharma LLC
- AIVITA Biomedical, Inc.
- Northwest Biotherapeutics, Inc.
- AstraZeneca
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
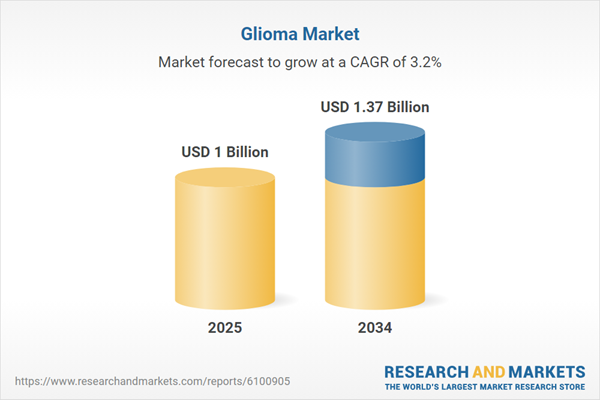
| Estimated Market Value ( USD | $ 1 Billion |
| Forecasted Market Value ( USD | $ 1.37 Billion |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |