Neuroendocrine Tumor Market Overview
Neuroendocrine tumors (NETs) are cancers that begin in specialised cells called neuroendocrine cells. They are rare and can occur in any body part. Most neuroendocrine tumors occur in the lungs, appendix, small intestine, rectum, and pancreas. The condition has symptoms such as fatigue, stomach pain, diarrhea, nausaea and vomiting, shortness of breath, and coughing. Neuroendocrine tumors are of different types such as functional NETs and non-functional NETs. They are also classified depending upon where the tumor is occurring in the body such as gastrointestinal neuroendocrine tumors (GI-NETs). Lung neuroendocrine tumours start in the lungs or bronchi and are the second most common type of NET. Pancreatic neuroendocrine tumors (P-NETs) are rare and occur in the pancreas and are the third most common type of NET.Neuroendocrine Tumor Market Growth Drivers
Increasing Clinical Trials Contributing to Potential Treatment Options
Neuroendocrine tumours are rare but life-threatening requiring effective treatment options, creating a gap of unmet needs in the market. As a result, research and clinical trials are growing. For instance, in June 2024, the Neuroendocrine Tumor Research Foundation reported that a new first-in-human clinical trial at the National Institutes of Health in Bethesda, Maryland was conducted to explore the use of an antibody-drug conjugate (ADC), ADCT-701, in adults with neuroendocrine neoplasms (NENs). This includes neuroendocrine tumors (NETs) and neuroendocrine carcinomas (NECs). ADCs are targeted cancer therapies that consist of antibodies linked to a drug through a connector called a linker. The clinical trial is targeted to determine the quantity of maximum tolerated dose of the humanised antibody ADCT-701 in participants suffering from neuroendocrine neoplasms or malignant adrenocortical carcinoma. Favourable outcomes of this clinical trial are projected to bring new treatment options and expand the treatment landscape to meet the increasing neuroendocrine tumor market demand.Breakthrough Designations by the FDA Marking Significant Milestones in the Treatment Landscape
The market is driven by the increasing number of regulatory approvals by authorities such as the United States Food and Drug Administration, bolstering the treatment landscape of the disease. For instance, in March 2024, the FDA granted breakthrough therapy designation to AlphaMedixTM (212Pb-DOTAMTATE) for the treatment of advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in adults, marking it as the first targeted alpha therapy to receive this status. The therapy is yet to be approved by the FDA, however, the breakthrough designation shows the potential of the AlphaMedixTM (212Pb-DOTAMTATE) as an effective treatment option for patients with metastatic or inoperable somatostatin receptor-expressing GEP-NETs. This advancement in the field is projected to boost the market demand for effective treatment options in the forecast period.Neuroendocrine Tumor Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Rising Prevalence of the Condition
The rising prevalence of neuroendocrine tumors is significantly driving the market growth, projected to create improved diagnostic techniques like imaging technologies (CT, MRI, PET) and greater disease awareness.Increased Research and Clinical Trials
The market growth is attributed to the continuous research and increase in clinical trials to explore effective treatment modalities for the disease.Advances in Diagnostic Techniques
The growing advancement in diagnostic techniques for neuroendocrine tumors including advanced medical imaging such as Gallium-68 PET/CT scan technologies is bolstering market growth and improved patient outcomes.Targeted Therapies and Immunotherapies
Researchers and healthcare providers are focusing more on exploring and developing effective targeted immunotherapies to treat the disease and improve patient outcomes.Neuroendocrine Tumor Market Segmentation
Market Breakup by Treatment Type
- Chemotherapy
- Immunotherapy
- Surgery
- Radiation Therapy
- Others
Market Breakup by Tumor Site
- Lungs
- Pancreas
- Colon
- Small Intestine
- Others
Market Breakup by Route of Administration
- Oral
- Parenteral
- Others
Market Breakup by End User
- Hospitals
- Clinics
- Diagnostic Centers
- Research Laboratories
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Neuroendocrine Tumor Market Share
Market Share by Treatment Type to Witness Significant Growth
The market segmentation by treatment type includes chemotherapy, immunotherapy, surgery, radiation therapy, and others. The market share is dominated by the surgery segment due to being the most common and first-line treatment for localised neuroendocrine tumors. The early-stage NETs are often treated by surgical removal. Further, radiation therapy is gaining significant traction, especially Peptide Receptor Radionuclide Therapy (PRRT) which is a targeted form of therapy with escalating adoption rates among healthcare providers for the treatment of the disease.Neuroendocrine Tumor Market Analysis by Region
The United States dominates the market share due to the presence of an advanced healthcare infrastructure. The advanced healthcare system enables timely and effective treatment options leading to early diagnosis and access to appropriate treatments. Several clinical trials are exploring the potential treatments for the disease to bring more effective and targeted therapies into the market. Further, the regional market is significantly driven by the rising prevalence of the disease and growing awareness among patients and healthcare providers, contributing to early diagnosis and treatment. The presence of many leading biotech and pharmaceutical companies involved in NET therapies and robust, high-tech research centres is driving the rapid development of innovative treatments, bolstering regional market dominance.Leading players in the Neuroendocrine Tumor Market
The key features of the market report comprise patent analysis, clinical trial analysis, grants analysis, funding and investment analysis and strategic initiatives by the leading players. The major companies in the market are as follows:Novartis AG
Novartis is a Swiss pharmaceutical company specialising in drug development for various indications. The company has a strong portfolio in the neuroendocrine tumor (NET) domain, primarily focusing on targeted therapies and radioligand therapies including Lutathera (177Lu-DOTATATE), a targeted radioligand therapy for advanced NETs, and Sandostatin (octreotide), which manages hormone-related symptoms in NET patients.Pfizer Inc
Pfizer Inc., a leading research-based pharmaceutical and biomedical company, is involved in research and development activities to create drugs for global non-invasive glucose monitoring and other cardiovascular diseases.Boehringer Ingelheim International GmbH
Boehringer Ingelheim has a significant focus on oncology research, offering treatments aimed at neuroendocrine tumors through targeted therapies and immuno-oncology approaches to improve patient outcomes.Eli Lilly and Company
Eli Lilly is a leading company developing therapies like targeted treatments and biologics for neuroendocrine tumors, focusing on personalised medicines and research collaborations to advance novel oncology therapies.F-Hoffmann-La Roche Ltd
Also known as Roche, is a Swiss healthcare company focused on developing pharmaceuticals and diagnostics for the healthcare domain across the globe. Roche has an extensive portfolio providing innovative cancer therapies, including targeted biologics and diagnostic solutions, for neuroendocrine tumors, leveraging immunotherapy and molecular diagnostics for personalised treatment approaches.Thermo Fisher Scientific
Thermo Fisher Scientific is a scientific equipment provider, offering advanced therapies for treating neuroendocrine tumors. Its diagnostic assay for monitoring disease progression in patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs) received FDA approval in September 2023.Key Questions Answered in the Neuroendocrine Tumor Market
- What was the neuroendocrine tumor market value in 2024?
- What is the neuroendocrine tumor market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on treatment type?
- How is the market segmented based on the tumor site?
- How does segmentation by route of administration influence market dynamics?
- Who are the end-users in the market?
- What are the major factors aiding the neuroendocrine tumor market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major neuroendocrine tumor market trends?
- Which technology is expected to dominate the market?
- Which type will lead the market segment?
- Which treatment type will dominate the market share?
- Which tumor site is poised to lead the market share?
- Which route of administration is projected to lead the market share?
- Which end-user will lead the market?
- Who are the key players involved in the neuroendocrine tumor market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Novartis AG
- Pfizer Inc.
- Boehringer Ingelheim International GmbH
- Eli Lilly and Company
- F-Hoffmann-La Roche Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
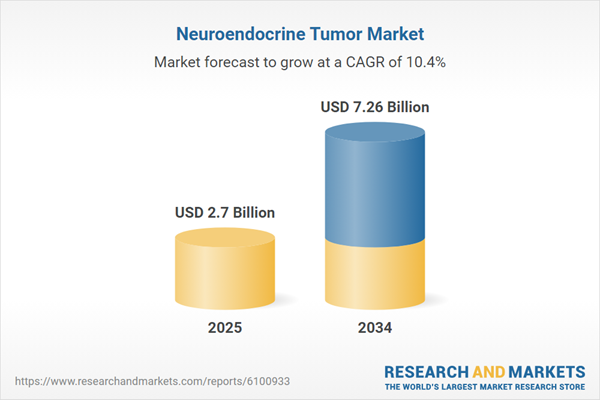
| Estimated Market Value ( USD | $ 2.7 Billion |
| Forecasted Market Value ( USD | $ 7.26 Billion |
| Compound Annual Growth Rate | 10.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |