Autologous Stem Cell and Non Stem Cell Therapies Market Overview
Autologous stem cell therapies involve using a patient's stem cells to treat various conditions, promoting tissue repair and regeneration. Non-stem cell therapies, on the other hand, include treatments like biologics, gene therapies, and monoclonal antibodies that target specific diseases. Both types are gaining momentum for their effectiveness in treating chronic, autoimmune, and degenerative conditions, driving market growth.Autologous Stem Cell and Non Stem Cell Therapies Market Growth Drivers
Continuous Advancements Positively Impacting Market Value
The growing demand for personalised treatments and advancements in regenerative medicine are key drivers supporting the growth of the market. For instance, in November 2024, GIOSTAR, a leader in stem cell research, announced that the U.S. FDA had cleared its IND application for a Phase-2 clinical trial using autologous mesenchymal stem cells to treat Type II diabetes. This innovative approach offers a promising solution to alleviate disease-induced damage, presenting a new treatment alternative with minimal side effects. The approval is poised to accelerate the adoption of autologous stem cell therapies in the diabetes treatment space, contributing to market expansion during the forecast period.Stem Cell and Gene Therapy Innovations to Impact the Autologous Stem Cell and Non Stem Cell Therapies Market Size Positively
Key market drivers such as advancements in gene therapy and the growing need for rare disease treatments are propelling the non-stem cell therapy market forward. For instance, in March 2024, Orchard Therapeutics (now part of Kyowa Kirin) received FDA approval for Lenmeldy (atidarsagene autotemcel) to treat early-onset metachromatic leukodystrophy (MLD) in children. This approval marks a significant milestone for gene therapy, offering a potential cure for a severe, rare disease. The entry of Lenmeldy into the market is expected to enhance growth in the gene therapy segment, boosting overall market development and accelerating the uptake of advanced non-stem cell treatments in the coming years.
Autologous Stem Cell and Non Stem Cell Therapies Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Advancements in Gene and Cell-Based Therapies for Non-Stem Cell Solutions
The market for non-stem cell therapies is witnessing substantial growth with the rise of gene and cell-based innovations. Advances in CRISPR and gene editing technologies are opening new therapeutic possibilities, offering treatments for genetic disorders, autoimmune diseases, and cancers. This trend is positioning non-stem cell therapies as essential alternatives to traditional treatment methods, driving market expansion and reshaping the therapeutic landscape.Preference for Personalised and Targeted Approaches to Boost Autologous Stem Cell and Non Stem Cell Therapies Market Demand
Personalised medicine, particularly in autologous stem cell therapies, is gaining prominence as treatments are increasingly tailored to individual genetic profiles and disease conditions. This approach improves patient outcomes by reducing the risk of complications and enhancing the effectiveness of therapies. As demand for precision healthcare rises, the market for autologous stem cell therapies is expected to expand, with increased adoption of personalised regenerative treatments.
Growing Demand for Minimally Invasive Non-Stem Cell Therapies
Minimally invasive non-stem cell therapies, such as biologic injections and platelet-rich plasma (PRP) treatments, are gaining traction for their effectiveness and reduced recovery times. These therapies provide patients with safer, less invasive options compared to traditional surgeries, driving the market growth. With a rising preference for less invasive procedures, non-stem cell therapies are expected to become an increasingly important component of the overall market.Increasing Use of Autologous Stem Cell Therapies for Chronic Diseases to Impact Autologous Stem Cell and Non Stem Cell Therapies Market Value Positively
A growing trend in the market is the increasing application of autologous stem cell therapies for the treatment of chronic diseases, such as osteoarthritis, cardiovascular diseases, and neurological disorders. These therapies are being used to regenerate damaged tissues and improve organ function. This shift towards regenerative solutions for long-term conditions is expanding the market potential, driving demand for advanced stem cell treatments.
Autologous Stem Cell and Non Stem Cell Therapies Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Type
- Autologous Stem Cell Therapies
- Autologous Non-Stem Cell Therapies
- CAR-T Cell Therapies
- Tumor Infiltrating Lymphocyte
Market Breakup by Indication
- Cancer Indications
- Musculoskeletal Indications
- Dermatological Indications
- Other Indications
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Cancer Centers
- Academic and Research Institutes
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Autologous Stem Cell and Non Stem Cell Therapies Market Share
Market Segmentation Based on Indication to Witness Significant Growth
Cancer indications are expected to hold the largest market share in the autologous stem cell and non-stem cell therapies market. This is due to the increasing prevalence of cancer globally, coupled with the effectiveness of stem cell-based treatments, such as CAR-T therapies, and non-stem cell solutions, like targeted immunotherapies, in improving patient outcomes.Autologous Stem Cell and Non Stem Cell Therapies Market Analysis by Region
Based on region, the market is divided into the United States, United Kingdom, Germany, France, Italy, Spain, Japan and India. Among these, the United States holds a significant market share due to advanced healthcare infrastructure, strong research and development capabilities, and high patient demand for innovative therapies. A favourable regulatory environment and substantial investments in biotechnology further support its dominance in the market.Leading Players in the Autologous Stem Cell and Non Stem Cell Therapies Market
The key features of the market report comprise patent analysis, clinical trials analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Gilead Sciences, Inc
Headquartered in Foster City, California, Gilead Sciences was established in 1987. The company focuses on advanced therapies, including autologous stem cell treatments, aimed at addressing cancer, viral diseases, and immunological conditions. Its portfolio includes cell therapies for oncology, along with an expanding range of non-stem cell therapies targeting chronic diseases and immune system disorders.Bristol-Myers Squibb Company
Founded in 1887 and based in New York, Bristol-Myers Squibb is a global biopharmaceutical company. The company is involved in both autologous stem cell and non-stem cell therapies, particularly in oncology and immunology. It develops innovative treatments, including CAR-T cell therapies and monoclonal antibodies, to address complex diseases like cancer, cardiovascular, and autoimmune disorders.Johnson & Johnson
Established in 1886 and headquartered in New Brunswick, New Jersey, Johnson & Johnson is a global leader in healthcare. The company’s portfolio spans a wide range of therapies, including autologous stem cell-based treatments for regenerative medicine and non-stem cell solutions like biologics and immunotherapies. Johnson & Johnson targets areas such as orthopaedics, neurology, and oncology with its innovative treatments.IOVANCE Biotherapeutics, Inc
Founded in 2011 and based in San Carlos, California, IOVANCE Biotherapeutics focuses on autologous T-cell therapies for cancer treatment. Specialising in tumour-infiltrating lymphocyte (TIL) therapies, the company is advancing treatments for melanoma, cervical cancer, and other solid tumours. Iovance's innovative approach aims to enhance the body’s immune response using personalised cell-based therapies to fight cancer.Other key players in the market include Vericel and Novartis AG.
Key Questions Answered in the Autologous Stem Cell and Non Stem Cell Therapies Market
- What was the autologous stem cell and non stem cell therapies market value in 2024?
- What is the autologous stem cell and non stem cell therapies market forecast outlook for 2025-2034?
- What is the market breakup based on the type?
- What is the market breakup based on the end user?
- What is the market breakup based on the indication?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major autologous stem cell and non stem cell therapies market trends?
- Which type will lead the market segment?
- Which end user will lead the market segment?
- Which indication will lead the market segment?
- Who are the key players involved in the autologous stem cell and non stem cell therapies market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Gilead Sciences, Inc.
- Bristol-Myers Squibb Company
- Johnson & Johnson
- IOVANCE Biotherapeutics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
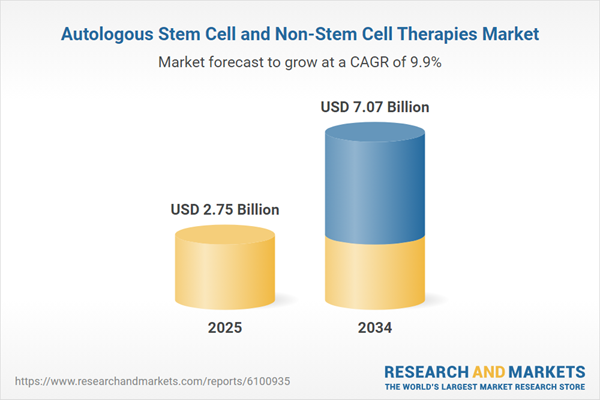
| Estimated Market Value ( USD | $ 2.75 Billion |
| Forecasted Market Value ( USD | $ 7.07 Billion |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |