Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market Overview
Acute lymphocytic/lymphoblastic leukaemia (ALL) is a rapidly progressing cancer of the blood and bone marrow, most commonly affecting children but also adults. The therapeutics market focuses on treatments like chemotherapy, targeted therapies, immunotherapy, and innovative treatments such as CAR T-cell therapy. Advances in drug development are improving survival rates and providing new hope for patients, driving market growth.Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market Growth Drivers
FDA Approvals Drive Market Growth
Increased research and clinical advancements in immunotherapy are pivotal drivers of the market growth. For instance, in June 2024, the U.S. Food and Drug Administration (FDA) approved Blinatumomab (Blincyto) for the treatment of adult and pediatric patients with CD19-positive Philadelphia chromosome-negative B-cell precursor ALL in the consolidation phase. This approval expands treatment options for ALL patients, providing a new therapeutic pathway. The approval of Blincyto is expected to boost market growth by offering more effective options, especially for those in advanced stages of the disease.Regulatory Advancements to Impact Acute Lymphocytic/Lymphoblastic Leukaemia Market Value Positively
The growing emphasis on personalised treatments and innovative cell-based therapies is driving the growth of the market. For instance, in November 2024, the FDA approved Obecabtagene Autoleucel (Aucatzyl), a genetically modified autologous T-cell immunotherapy, for relapsed or refractory B-cell precursor ALL in adults. This approval introduces a new treatment modality focused on advanced, hard-to-treat cases. The introduction of Aucatzyl is poised to significantly impact the market, offering new hope for patients and expanding therapeutic options in the ALL-treatment landscape, especially in challenging relapsed cases.Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Growth of CAR T-cell Therapy to Drive Market Growth
The introduction of chimeric antigen receptor (CAR) T-cell therapy has revolutionised the treatment of Acute Lymphocytic Leukemia (ALL). This innovative immunotherapy, which uses modified T-cells to target and destroy cancer cells, is showing significant promise in treating relapsed and refractory cases. Its growing adoption will likely lead to rapid market expansion and improved patient outcomes in the forecast period.Targeted Therapies and Personalised Medicine Boost Acute Lymphocytic/Lymphoblastic Leukemia Market Value
With a growing emphasis on personalised medicine, the Acute Lymphocytic Leukemia market is experiencing a shift towards targeted therapies. These therapies aim to attack cancer cells while minimizing damage to healthy tissues, improving efficacy and reducing side effects. As diagnostic techniques advance, precision medicine will become more widespread, driving demand for tailored treatments and contributing to the market's long-term growth.
Multi-Mechanism Drug Combinations Set to Expand Acute Lymphocytic/Lymphoblastic Leukemia Market Size
The combination of chemotherapy with novel biologic agents and immunotherapies is emerging as a key trend in ALL therapeutics. Studies show that multi-mechanism approaches may enhance therapeutic efficacy, reduce relapse rates, and improve survival outcomes. As clinical trials continue to support the effectiveness of these combinations, we anticipate an increase in approval of such therapies, driving market value and expanding treatment options.R&D Investments to Meet Rising Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market
The increasing investment in research and development (R&D) by pharmaceutical companies and biotechnology firms is accelerating the discovery of new treatments for ALL. With a focus on exploring novel drug candidates, including immune checkpoint inhibitors and monoclonal antibodies, R&D investments are expected to drive the development of next-generation therapies, pushing the market forward. Enhanced R&D is expected to broaden treatment options and fuel market growth in the forecast period.Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Treatment Type
- Drugs
- Oncaspar
- Hyper-CVAD Regimen
- CALGB 8811 Regimen
- Linker Regimen
- Others
- Therapy
- Chemotherapy
- Radiation Therapy
- Immunotherapy
- Others
Market Breakup by Route of Administration
- Oral
- Parenteral
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Academic and Research Institutes
- Cancer Centers
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market Share
Market Segmentation Based on Treatment Type to Witness Significant Growth
The drug segment leads the therapeutics market due to the high demand for targeted therapies and innovative treatments like immunotherapy and chemotherapy regimens. Oncaspar, a key drug for ALL, is frequently used to treat relapsed or refractory cases, contributing significantly to market share. Additionally, the ongoing development of new therapeutic drugs, including monoclonal antibodies and CAR T-cell therapies, is driving growth in this segment, as they offer more effective, personalised treatment options with fewer side effects compared to traditional chemotherapy.Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market Analysis by Region
The United States leads the therapeutics market due to its advanced healthcare infrastructure, high healthcare expenditure, and early adoption of innovative therapies such as CAR T-cell therapy and monoclonal antibodies. The presence of key pharmaceutical companies and strong clinical research activities further strengthens the market. Additionally, the high incidence of ALL and favourable reimbursement policies contribute to the U.S. holding the largest market share in this sector.Leading Players in the Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market
The key features of the market report comprise patent analysis, clinical trials analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:ERYtech Pharma
ERYtech Pharma, based in Lyon, France, was established in 2004. The company focuses on developing innovative therapies for acute lymphocytic leukaemia (ALL) and other cancers. Its lead product, Graspa, is an enzyme-based therapy for treating ALL, currently undergoing clinical trials. ERYtech Pharma's portfolio also includes products targeting metabolic diseases and cancer, expanding its presence in the oncology therapeutics market.Pfizer Inc
Pfizer Inc., headquartered in New York, USA, was founded in 1849. A global leader in biopharmaceuticals, Pfizer has a significant presence in the acute lymphocytic leukaemia (ALL) market. Their portfolio includes chemotherapy agents and targeted therapies like inotuzumab ozogamicin, a treatment for relapsed/refractory ALL. Pfizer is also exploring novel immunotherapies and CAR T-cell treatments to advance ALL care.Amgen Inc
Amgen Inc., established in 1980 and headquartered in Thousand Oaks, California, is a major player in the oncology therapeutics market, including treatments for acute lymphocytic leukaemia (ALL). Amgen’s portfolio features biologic therapies, including Blincyto® (blinatumomab), a bispecific T-cell engager for relapsed/refractory ALL. The company is committed to advancing treatment options for haematologic malignancies through extensive R&D efforts.Novartis AG
Novartis AG, founded in 1996 and headquartered in Basel, Switzerland, is a global leader in the development of therapies for haematologic diseases, including acute lymphocytic leukaemia (ALL). The company’s portfolio includes Kymriah® (tisagenlecleucel), a CAR T-cell therapy approved for relapsed/refractory ALL. Novartis is also expanding its pipeline with novel therapies targeting immune checkpoints and other mechanisms for ALL treatment.Other key players in the market include Rare Disease Therapeutics, Inc., Baxter International Inc., and Astellas Pharma Inc
Key Questions Answered in the Acute Lymphocytic/Lymphoblastic Leukemia Therapeutics Market
- What was the acute lymphocytic/lymphoblastic leukemia therapeutics market value in 2024?
- What is the acute lymphocytic/lymphoblastic leukemia therapeutics market forecast outlook for 2025-2034?
- What is the market breakup based on the treatment type?
- What is the market breakup based on the route of administration?
- What is the market breakup based on the end user?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major acute lymphocytic/lymphoblastic leukemia therapeutics market trends?
- Which treatment type will lead the market segment?
- Which route of administration will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the venous ulcer market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- ERYtech Pharma
- Pfizer Inc.
- Amgen Inc
- Novartis AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
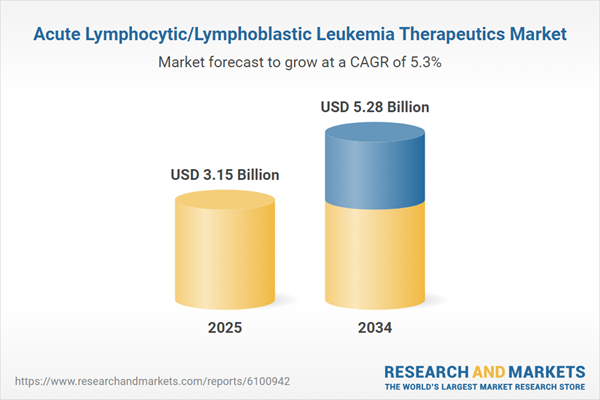
| Estimated Market Value ( USD | $ 3.15 Billion |
| Forecasted Market Value ( USD | $ 5.28 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |