United States Osteoarthritis Therapeutics Market Overview
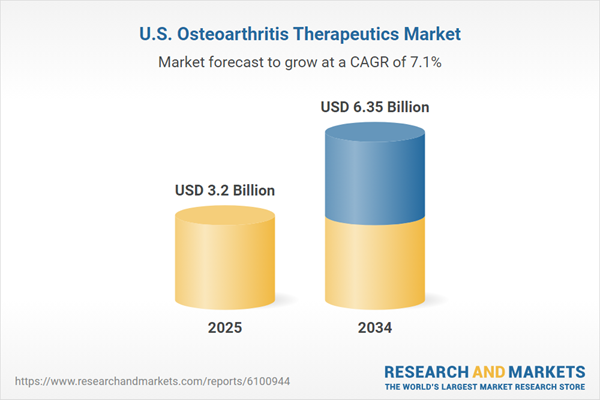
Osteoarthritis therapeutics include a range of treatments such as analgesics, nonsteroidal anti-inflammatory drugs, corticosteroid injections, physical therapy, and surgical options. These aim to relieve joint pain, reduce inflammation, enhance mobility, and slow the progression of cartilage degradation. The market was valued at USD 3.20 Billion in 2024 and is expanding due to an aging population, increasing obesity, and growing awareness of early treatment. Continued advancements in drug development and patient care are expected to drive sustained growth.United States Osteoarthritis Therapeutics Market Growth Drivers
Increasing Prevalence of Osteoarthritis to Accelerate the Market Growth
The substantial and rising prevalence of osteoarthritis (OA) in the United States continues to be a major driver of growth in the osteoarthritis therapeutics market. According to Michael Langworthy et al., 2024, osteoarthritis affects approximately 9,961 individuals per 100,000 in the United States, with the overall lifetime risk of developing symptomatic osteoarthritis ranging between 41% and 45%. This high and increasing disease burden is fostering sustained demand for advanced therapeutic options, thereby accelerating market expansion.United States Osteoarthritis Therapeutics Market Trends
Some of the notable trends in the market are surge in FDA approvals and a growing focus on government initiatives:Rising FDA Approvals to Accelerate the Growth in the Market
The market is witnessing robust growth, largely driven by an increasing number of FDA approvals for novel treatments. A landmark development occurred in May 2023, when Grünenthal’s resiniferatoxin (RTX) received Breakthrough Therapy Designation from the U.S. Food and Drug Administration for pain associated with knee osteoarthritis. This designation highlights the promising potential of RTX as a non-opioid therapy and is expected to expedite its development, offering hope for effective pain relief and advancing the market further.Rising Initiatives to Make Novel Treatments Accessible to Patients to Drive United States Osteoarthritis Therapeutics Market Growth
The government is actively involved in bring the latest treatment alternatives to the patients. For instance, the government launched the NITRO program under ARPA-H in May 2023. The project was aimed at driving groundbreaking innovation in osteoarthritis (OA) treatment. The program focused on developing regenerative therapies to repair damaged joints, potentially reducing the need for invasive surgeries like joint replacements. Such initiatives are expected to significantly enhance market growth and offer a more effective solution for managing osteoarthritis, improving patients' quality of life, and addressing the healthcare burden associated with the condition.
United States Osteoarthritis Therapeutics Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Type
- Hip Osteoarthritis
- Spinal Osteoarthritis
- Knee Osteoarthritis
- Hand Osteoarthritis
- Others
Market Breakup by Drug Class
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
- Other Analgesics
- Corticosteroids
- Hyaluronic Acid Injections
- Others
Market Breakup by Dosage Form
- Tablets and Capsules
- Injections
- Creams and Gels
- Others
Market Breakup by Route of Administration
- Oral
- Parenteral
- Topical
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Homecare Settings
- Others
Market Breakup by Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
United States Osteoarthritis Therapeutics Market Share
NSAIDs Segment Based on Drug Class to Witness Substantial Growth
Based on drug class, the market is segmented into nonsteroidal anti-inflammatory drugs (NSAIDs), other analgesics, corticosteroids, hyaluronic acid injections, and others. The NSAIDs segment is projected to dominate the market, attributed to the well-established efficacy of NSAIDs in reducing inflammation and relieving joint pain, and their widespread availability in both prescription and over-the-counter forms. Additionally, their relatively low cost and strong physician preference further contribute to their dominant market share.Leading Players in the United States Osteoarthritis Therapeutics Market
The key features of the market report comprise clinical trials analysis, patent analysis, grant analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Bayer AG
Bayer AG, established in 1863 and headquartered in Leverkusen, Germany, is a leading company in the market. Its product, Aleve (Naproxen Sodium), is a well-known pain reliever used in the treatment of osteoarthritis. Bayer has been involved in clinical studies, including trials for assessing treatment responsiveness to medications like naproxen for osteoarthritis stiffness. These initiatives highlight Bayer’s commitment to advancing osteoarthritis care and pain management solutions for patients in the United States.Pfizer Inc
Headquartered in New York City and established in 1849, Pfizer is a leading pharmaceutical company with a notable presence in the United States market. Pfizer has been involved in the clinical development of tanezumab, an investigational non-opioid monoclonal antibody targeting nerve growth factor (NGF). The Phase 3 trial results published in 2018 demonstrated significant improvements in pain relief and physical function for osteoarthritis patients, highlighting Pfizer’s commitment to addressing unmet needs in OA treatment through innovative biologic therapies.Eli Lilly and Company
Eli Lilly and Company is a global pharmaceutical leader actively involved in advancing osteoarthritis treatment in the United States. The company is committed to addressing chronic musculoskeletal conditions through innovative research and non-opioid therapeutic development. The company is actively developing LY3526318, a novel non-opioid drug targeting chronic osteoarthritis knee pain. Through continued innovation and focused therapeutic research, Lilly reinforces its strong commitment to addressing the growing burden of osteoarthritis.Pacira Biosciences Inc
Headquartered in Tampa, Florida, and established in 2007, Pacira BioSciences Inc. is a prominent American biopharmaceutical company specializing in non-opioid pain management. In March 2024, Pacira’s gene therapy candidate PCRX-201 received the FDA’s Regenerative Medicine Advanced Therapy (RMAT) designation for osteoarthritis of the knee, the first gene therapy to do so in this indication. Supported by positive Phase 1 data, PCRX-201 shows potential as a disease-modifying treatment. Alongside this innovation, Pacira continues to market Zilretta, an extended-release corticosteroid for osteoarthritis knee pain, reinforcing its leadership in the market.Other key players in the market include Sanofi S.A., Bristol Myers Squibb, Assertio Holdings Inc., Anika Therapeutics Inc., and Bioventus LLC.
Key Questions Answered in the United States Osteoarthritis Therapeutics Market Report
- What was the United States osteoarthritis therapeutics market value in 2024?
- What is the United States osteoarthritis therapeutics market forecast outlook for 2025-2034?
- What are the major factors aiding the United States osteoarthritis therapeutics market demand?
- How has the market performed so far, and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major United States osteoarthritis therapeutics market trends?
- Which type is expected to dominate the market segment?
- Which drug class is projected to lead the market segment?
- Which dosage form is projected to lead the market segment?
- Which route of administration is anticipated to drive the market segment?
- Which end user is anticipated to drive the market segment?
- Which distribution channel is likely to dominate the market segment?
- Who are the key players involved in the United States osteoarthritis therapeutics market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Bayer AG
- Pfizer Inc.
- Eli Lilly and Company
- Pacira Biosciences Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 3.2 Billion |
| Forecasted Market Value ( USD | $ 6.35 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | United States |
| No. of Companies Mentioned | 4 |