United States Heart Valve Devices Market Overview
Heart valve devices refer to medical devices designed to repair or replace damaged or diseased heart valves. These devices are used for the treatment of conditions such as aortic stenosis, mitral regurgitation, and tricuspid regurgitation. The rising advancements in technology, increasing prevalence of cardiovascular diseases, and favorable regulatory environments are some of the major drivers of the United States market for heart valve devices. Moreover, the country benefits from favorable regulatory frameworks and reimbursement policies that encourage the adoption of advanced heart valve devices, which is likely to fuel the market growth in the forecast period.United States Heart Valve Devices Market Growth Drivers
Rising Prevalence of Cardiovascular Diseases to Support Market Growth
Cardiovascular diseases (CVDs), including heart disease, stroke, and heart valve diseases, are becoming increasingly prevalent due to lifestyle factors such as poor diet, lack of exercise, smoking, and excessive alcohol consumption. In 2022, coronary heart disease (CHD) accounted for 39.5% of all deaths attributable to cardiovascular diseases in the United States, followed by stroke at 17.6%, and other CVD at 17%, according to the American Heart Association. The growing prevalence of these conditions is driving the demand for heart valve devices to treat and manage valve-related diseases, which is anticipated to support market expansion in the country in the coming years.United States Heart Valve Devices Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Rising Investment to Support Heart Valve Device Innovation
In December 2024, Capstan Medical, a California-based company developing heart valve implants using a robotic delivery system, announced it had raised USD 110 million in new funding to support its progress toward pivotal clinical trials. The company’s mitral valve technology is expected to enter first-in-human trials in early 2025, with pivotal trials planned for 2026. This significant investment reflects the growing interest and financial support in the United States heart valve devices market.Increasing Regulatory Approvals
One of the major market trends is the increasing regulatory approvals of heart valve devices, allowing innovative solutions to address critical heart conditions. For instance, in April 2024, the U.S. Food and Drug Administration (FDA) granted approval to Abbott's TriClip™ transcatheter edge-to-edge repair (TEER) system, the first device specifically designed to treat tricuspid regurgitation (TR), or a leaky tricuspid valve. These regulatory approvals are expected to accelerate product development, expand access to advanced cardiovascular treatments, and meet the United States heart valve devices market demand.United States Heart Valve Devices Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Valve Type
- Biological (Tissue) Valve
- Mechanical Valve
Market Breakup by Product Type
- Replacement Devices
- Mechanical Valve
- Bioprosthetic Valve
- Transcatheter Aortic Valve Replacement (TAVR)
- Repair Devices
- Surgical Valve
- Transcatheter Mitral Valve Replacement (TMVR)
- Balloon Valvuloplasty Devices
Market Breakup by Procedure
- Open Surgery
- Minimally Invasive Surgery (MIS)
Market Breakup by End User
- Hospitals
- Ambulatory Surgical Centers (ASCs)
- Others
United States Heart Valve Devices Market Share
Segmentation Based on Valve Type to Witness Substantial Growth
Based on the valve type, the market is segmented into biological (tissue) valve and mechanical valve. In the United States, the biological (tissue) valve segment covers a substantial market share, which is primarily due to the valves' natural composition that reduces the need for long-term anticoagulation therapy compared to mechanical valves. Moreover, advancements in tissue engineering and preservation are improving the durability and performance of biological valves, thereby boosting their market demand.United States Heart Valve Devices Market Analysis by Region
In the United States, the Northeast and West Coast regions hold a high market value due to advanced healthcare infrastructure and higher adoption rates of innovative treatments like transcatheter aortic valve replacement (TAVR). The Midwest and Southern states are witnessing a growing demand, owing to the expanding aging population and increasing prevalence of cardiovascular diseases, which are fueling the need for heart valve treatments. Moreover, the presence of major medical device companies and healthcare hubs in states like California, Massachusetts, and Minnesota is expected to boost market growth in the region.Leading Players in the United States Heart Valve Devices Market
The key features of the market report comprise patent analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Medtronic plc
Medtronic is a leading player in the heart valve devices market of the United States, with its TAVR systems being widely used for treating aortic stenosis. The company is focused on accelerating innovation in minimally invasive heart valve therapies. Medtronic offers a wide range of heart valve devices, including the Evolut™ R and Evolut™ PRO transcatheter aortic valve replacement (TAVR) systems, along with surgical heart valves and repair technologies.Edwards Lifesciences Corporation
Edwards Lifesciences, headquartered in Irvine, California, specializes in artificial heart valves and hemodynamic monitoring. The company's portfolio includes transcatheter heart valves (THVs), including the SAPIEN™ series for aortic valve replacement. It also offers surgical heart valves and critical care monitoring devices, and significantly contributes to the United States heart valve devices market growth.Abbott
Abbott, with its headquarters in Illinois (United States), has a prominent presence in the market. The company’s structural heart portfolio includes the MitraClip™ device for mitral valve repair, the TriClip™ device for tricuspid valve repair, and the Portico™ TAVR system for aortic valve replacement. Abbott engages in strategic acquisitions and product innovations to expand its footprint in the United States heart valve devices market.Corcym Group
Corcym Group, a global medical device company specializing in the structural heart area, is one of the key players in the market. The company specializes in mechanical and biological heart valves, including the Carbomedics™ and Bicarbon™ series. While Corcym is based in Italy, its heart valve products are distributed in the United States through partnerships with local medical device companies.Other key players in the market include Boston Scientific Corporation, Artivion, Inc., Shockwave Medical Inc. (Johnson & Johnson Services, Inc.), JenaValve Technology, Inc., and Micro Interventional Devices, Incorporated.
Key Questions Answered in the United States Heart Valve Devices Market Report
- What was the United States heart valve devices market value in 2024?
- What is the United States heart valve devices market forecast outlook for 2025-2034?
- What is the market segmentation based on valve type?
- What is the market segmentation based on product type?
- What is the market breakup based on the procedure?
- What is the market breakup by end user?
- What major factors aid the United States heart valve devices market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the major drivers, opportunities, and restraints in the market?
- What are the major trends influencing the market?
- Who are the key players involved in the United States heart valve devices market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Medtronic plc
- Edwards Lifesciences Corporation
- Abbott
- Corcym Group
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
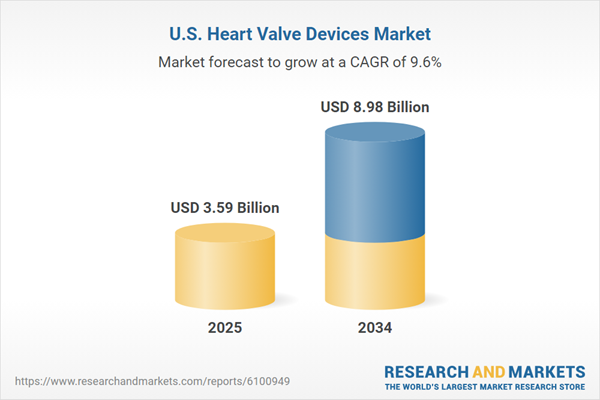
| Estimated Market Value ( USD | $ 3.59 Billion |
| Forecasted Market Value ( USD | $ 8.98 Billion |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | United States |
| No. of Companies Mentioned | 4 |