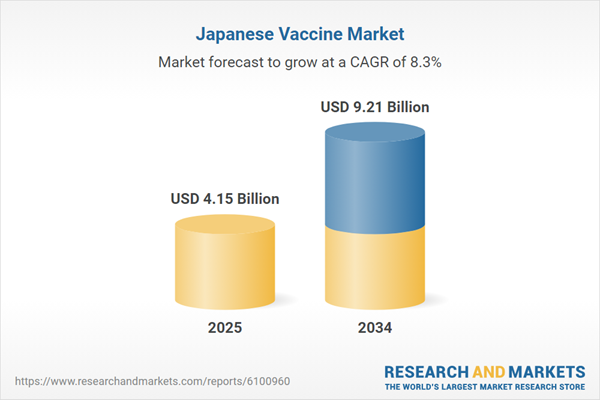
Japan Vaccine Market Overview
A vaccine is a biological substance that stimulates the immune system to recognize and fight specific pathogens, providing immunity against diseases. The market is driven by increasing awareness, government initiatives, and rising healthcare investments. It is expected to grow due to a high demand for preventive healthcare and advancements in vaccine technology. Japan's aging population further boosts the market’s expansion.Japan Vaccine Market Growth Drivers
Introduction of Innovative Vaccine Technologies to Boost Market Demand
The introduction of innovative vaccine technologies is poised to drive market growth. For instance, in January 2024, Japan prepared for the launch of ARCT-154, the first fully approved sa-mRNA COVID-19 vaccine. Co-developed by Arcturus Therapeutics and CSL, this vaccine offers safer, scalable production, potentially enhancing Japan's vaccination efforts and accelerating market growth.Japan Vaccine Market Trends
The market is witnessing several trends, including expanding regulatory support coupled with a growing emphasis on enhancing vaccine distribution and accessibility.Regulatory Support to Boost Japan Vaccine Market Growth
The approval of new vaccines is a major trend that plays a vital role in expanding the vaccine market in Japan. In May 2024, the World Health Organization (WHO) approved Takeda’s QDENGA® dengue vaccine, which is set to make a significant impact on Japan’s vaccine industry. This approval makes QDENGA a key player in preventing dengue, especially in areas with high transmission. Its potential for global use and inclusion in public health programs highlights Japan’s growing role in the vaccine market, driving further growth and innovation.Increasing Focus on Vaccine Distribution and Accessibility to Elevate Japan Vaccine [Market Value
A significant trend in the market is the increasing collaboration for vaccine distribution and accessibility, particularly to combat preventable diseases. For instance, according to August 2024 data, UNICEF, with support from Japan, delivered around 60,000 doses of the PENTA-Hib vaccine. This effort, coupled with Japan’s investment in vaccine infrastructure, is expected to drive further market growth and strengthen global vaccination initiatives in the years ahead.Japan Vaccine Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Product Type
- Multivalent Vaccines
- Monovalent Vaccines
Market Breakup by Type
- Subunit Vaccines
- Recombinant vaccines
- Conjugate Vaccines
- Toxoid vaccines
- Inactivated
- Live Attenuated
- mRNA vaccines
- Viral vector vaccines
Market Breakup by Route of Administration
- Oral
- Parenteral
- Nasal
Market Breakup by Disease Indication
- Viral Diseases
- Hepatitis
- Influenza
- HPV
- MMR
- Rotavirus
- Herpes Zoster
- COVID-19
- Others
- Bacterial Vaccines
- Meningococcal Diseases
- Pneumococcal diseases
- DPT
- Others
- Cancer Vaccines
- Allergy Vaccines
Market Breakup by Age
- Pediatric
- Adult
Market Breakup by End User
- Hospital Pharmacies
- Retail Pharmacies
- Government Suppliers
- Others
Japan Vaccine Market Share
Segmentation Based on Application to Witness Substantial Growth
By type, the market is divided into subunit vaccines, inactivated, live attenuated, mRNA vaccines, and viral vector vaccines. Among the vaccine types, mRNA vaccines are expected to hold a substantial share of the market. Their rapid development capabilities, high efficacy, and adaptability to emerging variants have positioned them at the forefront of vaccine innovation. Continued investment in research and strong support from regulatory bodies further drive their growth, especially in the context of pandemic preparedness and personalized medicine.Japan Vaccine Market Analysis by Region
The Japan vaccine market demonstrates strong regional growth, particularly in Eastern and Northern Japan. Eastern Japan, including Tokyo and surrounding prefectures, holds a market share due to advanced healthcare infrastructure and high immunization awareness. Northern Japan shows increasing market demand driven by government initiatives and rising healthcare investments. These regions collectively contribute significantly to the market's expansion and ongoing public health improvements.Leading Players in the Japan Vaccine Market
The key features of the market report comprise patent analysis, clinical trials analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:GlaxoSmithKline plc
GSK (GlaxoSmithKline plc), established in 2000 and headquartered in Brentford, UK, is a leading global biopharmaceutical company. In September 2023, it received approval from Japan’s Ministry of Health, Labour and Welfare for Arexvy, the first respiratory syncytial virus (RSV) vaccine for older adults. This vaccine addresses the growing need to protect adults aged 60 and above, particularly those with underlying health conditions. GSK continues to expand its vaccine portfolio in Japan, significantly impacting public health.Merck & Co.
Merck & Co., Inc. is a global healthcare leader which was established in 1891. In the Japan vaccine market, Merck plays a key role with its GARDASIL®9 vaccine, which targets HPV-related cancers. In September 2024, the company reported positive Phase 3 trial results for GARDASIL®9 in Japanese males, reinforcing its commitment to addressing HPV-related health concerns in Japan.
Sanofi
Sanofi, headquartered in Paris, France, focuses on transforming medicine with innovative treatments and vaccines. In May 2024, Sanofi entered a co-exclusive licensing agreement with Novavax to commercialize COVID-19 vaccines and develop a flu-COVID-19 combination vaccine. This partnership will enhance access to vaccines in the Japan market, offering broader protection against respiratory diseases.Pfizer Inc
Pfizer is a leading pharmaceutical company specializing in the development of innovative medicines and vaccines. Notably, Pfizer, in collaboration with BioNTech, has supplied the Omicron XBB.1.5-adapted COVID-19 vaccine (COMIRNATY) to Japan. This vaccine, based on mRNA technology, is pivotal in Japan's 2023 Autumn Special Vaccination Program, showcasing Pfizer's significant role in the Japan vaccine market growth.Other key players in the market include CSL Behring, AstraZeneca plc, and Johnson & Johnson Services, Inc
Key Questions Answered in the Japan Vaccine Market Report
- What was the Japan vaccine market value in 2024?
- What is the Japan vaccine market forecast outlook for 2025-2034?
- What is the market segmentation based on product type?
- What is the market segmentation based on type?
- What is the market breakup based on the route of administration?
- How is the market segmented based on disease indication?
- How is the market segmented based on age?
- How is the market divided based on the end user?
- What are the major factors aiding the Japan vaccine market demand?
- How has the market performed so far, and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major Japan vaccine market trends?
- Which product type is expected to dominate the market segment?
- Which type is expected to dominate the market segment?
- Which route of administration is projected to lead the market segment?
- Which disease indication is projected to lead the market segment?
- Which age is anticipated to drive the market segment?
- Which end user is likely to dominate the market segment?
- Who are the key players involved in the Japan vaccine market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- GlaxoSmithKline plc
- Merck & Co.
- Sanofi
- Pfizer Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 4.15 Billion |
| Forecasted Market Value ( USD | $ 9.21 Billion |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Japan |
| No. of Companies Mentioned | 4 |