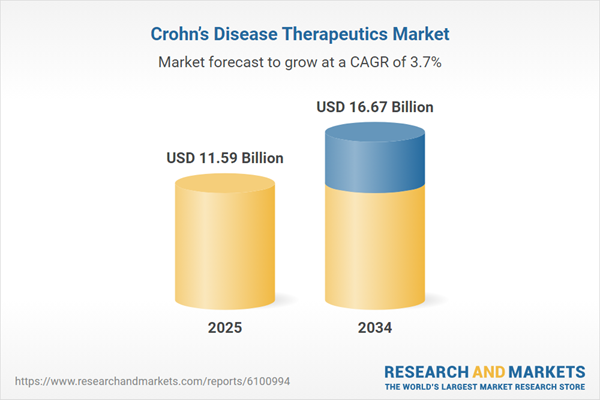
Crohn’s Disease Therapeutics Market Overview
Crohn’s disease is a chronic inflammatory bowel disease (IBD) that causes inflammation of the digestive tract, leading to symptoms such as abdominal pain, diarrhea, fatigue, and weight loss. The condition can occur anywhere in the gastrointestinal tract. Therapeutics include anti-inflammatory drugs, immunosuppressants, biologics, and surgical interventions, aiming to manage symptoms, reduce inflammation, and improve quality of life.Crohn’s Disease Therapeutics Market Growth Drivers
Surge in Clinical Trials to Boost Market Growth
The increasing demand for novel, targeted therapies and the rise in Crohn’s disease prevalence are significant drivers for the market. For instance, in October 2024, Abivax SA announced the enrolment of the first patient in its Phase 2b ENHANCE-CD trial to evaluate obefazimod in patients with moderate to severe Crohn's disease. This trial, focusing on induction and maintenance therapy, aims to assess the drug’s efficacy and safety. Obefazimod could offer a new oral treatment for patients who have not responded to conventional therapies. The positive results from this trial would significantly impact the market by providing an additional effective therapy option, driving growth in the coming years.Growth in Approvals from Regulatory Authorities to Meet Increasing Crohn’s Disease Therapeutics Market Demand
The rising demand for affordable biologics and increased healthcare accessibility are accelerating the growth of biosimilar products. For instance, in December 2024, Biocon Biologics received US FDA approval to launch its biosimilar version of Janssen’s Stelara (Ustekinumab) for autoimmune disorders, including Crohn’s disease. With the approval of its biosimilar, Yesintek, Biocon is poised to compete with established brands in the market. The upcoming launch of this biosimilar, expected by February 2025, will likely reduce treatment costs and increase market access for Crohn's disease therapies, fostering competitive pricing and growth in the therapeutic market during the forecast period.
Crohn’s Disease Therapeutics Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Increasing Adoption of Biologics and Precision Medicine to Fuel Market Expansion
The market for Crohn's disease therapeutics is increasingly shifting towards biologic treatments, with monoclonal antibodies gaining popularity. These targeted therapies, such as TNF inhibitors and interleukin inhibitors, offer more effective and personalised treatments, reducing inflammation and improving patients' quality of life. This trend is expected to drive significant growth in the market, particularly as new biologics are approved.Increased Focus on Early Diagnosis and Precision Medicine to Boost Crohn’s Disease Therapeutics Market Value
As early diagnosis and better disease management become key priorities in treating Crohn’s disease, there is a surge in advanced diagnostic tools and treatment options. Precision medicine, combined with biomarkers, is enhancing early intervention strategies, enabling timely treatment to reduce complications and hospitalisations. This trend is poised to positively influence market development in the forecast period.
Rise of Oral Therapeutics to Meet Increasing Crohn’s Disease Therapeutics Market Demand
Oral therapeutics are gaining traction in the Crohn’s disease therapeutics market as patients seek more convenient treatment options. Innovative oral formulations, such as oral biologics and small molecules, are becoming available, reducing the need for injections or infusions. This trend is likely to support market expansion as patients and healthcare providers prioritise ease of use and adherence.Adoption of Biosimilars to Impact the Crohn’s Disease Therapeutics Market Size Positively
The rise of biosimilars is significantly impacting the Crohn’s disease market by providing cost-effective alternatives to high-priced biologics. As patents for several blockbuster biologics expire, biosimilars offer an opportunity for reduced treatment costs, improving access for a larger patient base. This trend is expected to boost market growth, especially in emerging regions with cost-sensitive populations.Crohn’s Disease Therapeutics Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Treatment Type
- Medication
- Anti-Inflammatory Agents
- Immunosuppressants
- Antibiotics and Antipyretics
- Other medications
- Nutritional Therapy
- Surgery
Market Breakup by Type
- Ileocolitis
- Ileitis
- Granulomatous colitis
- Gastroduodenal Crohn’s Disease
- Jejunoileitis
- Perianal Crohn’s Disease
Market Breakup by Route of Administration
- Oral
- Parenteral
- Others
Market Breakup by End User
- Hospital
- Ambulatory Surgical Centers
- Specialty Clinics
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Crohn’s Disease Therapeutics Market Share
Ileocolitis is expected to hold the largest market share in the forecast period. The market share can be attributed to its higher prevalence, affecting both the ileum and colon. This form is more commonly diagnosed, leading to a greater demand for therapies focused on managing inflammation, controlling symptoms, and preventing flare-ups, contributing to its dominant position in the market.Crohn’s Disease Therapeutics Market Analysis by Region
The United States holds the largest market share for Crohn’s disease therapeutics due to high disease prevalence, advanced healthcare infrastructure, and strong access to innovative biologic treatments. The presence of leading pharmaceutical companies and increasing demand for personalised therapies further contribute to the region’s dominance in the market.Leading Players in the Crohn’s Disease Therapeutics Market
The key features of the market report comprise patent analysis, clinical trials analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Takeda Pharmaceutical Company Limited
Takeda Pharmaceutical Company Limited, headquartered in Tokyo, Japan, was established in 1781. The company is a global leader in immunology and gastroenterology, focusing on inflammatory bowel diseases, including Crohn's disease. Takeda's portfolio includes Entyvio (vedolizumab), a biologic approved for treating moderate-to-severe Crohn's disease, and other therapies aimed at improving patient outcomes in gastrointestinal disorders.AbbVie Inc
AbbVie Inc., founded in 2013 and headquartered in North Chicago, USA, is a global biopharmaceutical company. AbbVie's portfolio in the Crohn's disease therapeutics market includes Humira (adalimumab), a widely used biologic, and Rinvoq (upadacitinib), an oral therapy. The company focuses on immunology treatments, including for inflammatory bowel diseases like Crohn's disease.Pfizer Inc
Pfizer Inc., established in 1849 and headquartered in New York, USA, is a multinational biopharmaceutical company. Pfizer offers innovative treatments for Crohn's disease, such as Xeljanz (tofacitinib), an oral Janus kinase (JAK) inhibitor. The company continues to develop therapies targeting immune-mediated diseases, enhancing the options for Crohn's disease management.Ferring B.V.
Ferring B.V., based in Saint-Prex, Switzerland, was founded in 1950. The company focuses on biopharmaceuticals in gastroenterology and reproductive health. Ferring's portfolio in Crohn's disease includes products like Revestive (teduglutide), used for short bowel syndrome, a complication associated with Crohn’s disease, and other treatments aimed at improving gastrointestinal health.
Other key players in the market include Bristol-Myers Squibb Company, F. Hoffmann-La Roche Ltd, UCB S.A., Salix Pharmaceuticals, and Gilead Sciences Inc
Key Questions Answered in the Crohn’s Disease Therapeutics Market
- What was Crohn’s disease therapeutics market value in 2024?
- What is the Crohn’s disease therapeutics market forecast outlook for 2025-2034?
- What is the market breakup based on the treatment type?
- What is the market breakup based on type?
- What is the market breakup based on the route of administration?
- What is the market breakup based on the end user?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major Crohn’s disease therapeutics market trends?
- Which type will lead the market segment?
- Which route of administration will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the Crohn’s disease therapeutics market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Takeda Pharmaceutical Company Limited
- AbbVie Inc.
- Pfizer Inc.
- Ferring B.V.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 11.59 Billion |
| Forecasted Market Value ( USD | $ 16.67 Billion |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |