Meningitis Treatment Market Overview
Meningitis treatment depends on the cause. Bacterial meningitis requires immediate hospitalization and intravenous antibiotics, often with corticosteroids to reduce inflammation. Viral meningitis is usually milder and treated with rest, fluids, and pain relief. Antiviral drugs may be used in severe viral cases. Fungal and parasitic meningitis are treated with specific antifungal or antiparasitic medications. Supportive care, such as oxygen and fluids, is essential in all types to manage symptoms and prevent complications.Meningitis Treatment Market Growth Drivers
Multivalent Vaccine Approvals Supporting Meningitis Treatment Market Expansion
The market expansion is driven by the rising demand for broad-spectrum vaccines and continuous innovation in meningococcal disease prevention. For instance, in February 2025, GSK plc received approval from the US Food and Drug Administration for Penmenvy, a vaccine designed to protect individuals aged 10 to 25 against five major Neisseria meningitidis serogroups (A, B, C, W, and Y). The vaccine combines antigens from two of GSK’s established meningococcal vaccines and demonstrated strong safety and immune response in Phase III trials. This approval is expected to significantly enhance preventive options and boost growth in the global meningitis treatment market.Meningitis Treatment Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Rising Infection Rates to Accelerate Market Growth
The United States witnessed its highest number of meningococcal disease cases in a decade, with 438 confirmed and probable cases reported in 2023. This surge underscores the pressing need for enhanced vaccination strategies and early diagnostics. The increased disease burden is expected to fuel investment in meningitis treatment options, boosting overall market expansion in the coming years.Innovative Formulations Driving Meningitis Treatment Market Value in Developing Countries
In April 2025, a sustained-release formulation of flucytosine for cryptococcal meningitis entered Phase II trials in Malawi and Tanzania, led by DNDi and partners. The novel formulation offers simplified administration and improved patient adherence. This advancement supports improved access and outcomes in underserved regions, reinforcing precision treatment strategies and contributing to the global growth of the meningitis treatment market.Technological Innovations Elevating the Meningitis Treatment Market Demand
Technological advancements in vaccine formulation and diagnostic techniques are transforming meningitis treatment. New conjugate and multicomponent vaccines are enhancing immune responses, while rapid diagnostic tools enable earlier intervention. These innovations support better disease management and expand market opportunities by reducing mortality and improving patient outcomes, particularly in high-burden regions where meningitis remains a critical health concern.Government Initiatives to Influence the Meningitis Treatment Market Size Positively
Increasing government-led immunization programs and global health collaborations are driving the market. Expanded funding for vaccination, public awareness campaigns, and strategic partnerships with pharmaceutical companies ensures broader access to preventive care. These efforts strengthen healthcare infrastructure, improve early diagnosis and treatment rates, and support long-term market value through sustained demand and policy-backed healthcare interventions.Meningitis Treatment Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Causative Organism
- Bacterial
- Viral
- Fungal
- Parasitic
Market Breakup by Treatment Type
- Drug Type
- Antibiotics Agents
- Antiviral Agents
- Corticosteroids
- Others
- Vaccine Type
- Meningococcal Conjugate Vaccine
- Meningococcal Polysaccharide Vaccine
- Combination Vaccine
- Others
Market Breakup by Route of Administration
- Oral
- Parenteral
- Others
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Meningitis Treatment Market Share
Bacterial Segment to Lead the Segmentation by Causative Organism
The bacterial segment is expected to hold the largest market share in the meningitis treatment market due to its higher prevalence and severity. Bacterial meningitis is a life-threatening condition that requires urgent intervention with antibiotics, which fuels ongoing demand for treatment. Viral meningitis, although less severe, is still common and drives the market but to a lesser extent. Fungal and parasitic meningitis are rarer, resulting in a smaller market share. The bacterial segment will continue to dominate due to its critical health impact and robust treatment options.Meningitis Treatment Market Analysis by Region
The United States is poised to hold the largest market share for meningitis treatment due to its advanced healthcare infrastructure, high incidence of bacterial meningitis, and significant investment in medical research. European countries like the United Kingdom and Germany are also expected to witness prominent growth due to high vaccination coverage. Japan’s advanced healthcare system contributes to its market presence, while India’s growing healthcare access will drive future growth, albeit at a smaller scale.Leading Players in the Meningitis Treatment Market
The key features of the market report comprise patent analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:BIO-MED
BIO-MED is an Indian pharmaceutical company founded in 1967, headquartered in Uttar Pradesh. It specializes in the research, development, and manufacture of vaccines and injectable formulations. The company produces affordable meningitis vaccines, contributing significantly to disease prevention across developing countries through immunization initiatives and public health partnerships.Novartis AG
Novartis AG, established in 1996 and headquartered in Basel, Switzerland, is a global healthcare leader focused on innovative medicines. Its vaccine division, formerly active in meningitis prevention, developed key products targeting meningococcal strains. Novartis continues to support neurological and infectious disease treatment through research, partnerships, and legacy vaccine innovations.Pfizer, Inc
Pfizer, Inc., founded in 1849 and based in New York, USA, is a leading pharmaceutical firm with a strong portfolio in vaccines, including meningitis prevention. Its meningococcal vaccine range is widely used globally. Pfizer invests heavily in research, aiming to broaden protection against multiple meningococcal serogroups through combination vaccines.Sanofi
Sanofi, founded in 2004 and headquartered in Paris, France, is a global biopharmaceutical company actively involved in meningitis prevention. Through its vaccines division, Sanofi Pasteur, the company develops and distributes conjugate vaccines targeting various meningitis-causing strains, particularly in endemic regions. Its innovations support global immunization programs and public health initiatives.Other key players in the market include GSK, Merck & Co., Inc., and F. Hoffmann-La Roche Ltd
Key Questions Answered in the Meningitis Treatment Market Report
- What was the meningitis treatment market value in 2024?
- What is the meningitis treatment market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is the market segmentation based on causative organism?
- What is the market segmentation based on treatment type?
- What is the market segmentation based on the route of administration?
- What is the market breakup based on the end user?
- What major factors aid the meningitis treatment market demand?
- How has the market performed so far, and how is it anticipated to perform in the coming years?
- What are the major drivers, opportunities, and restraints in the market?
- What are the major trends influencing the market?
- Which regional market is expected to dominate the market share in the forecast period?
- Which country is likely to experience elevated growth during the forecast period?
- Who are the key players involved in the meningitis treatment market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- BIO-MED
- Novartis AG
- Pfizer, Inc.
- Sanofi
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
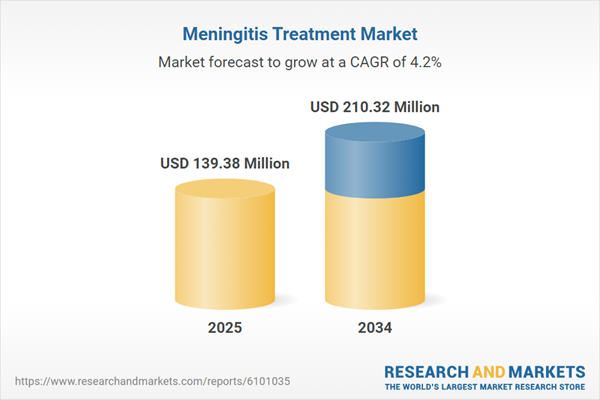
| Estimated Market Value ( USD | $ 139.38 Million |
| Forecasted Market Value ( USD | $ 210.32 Million |
| Compound Annual Growth Rate | 4.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |