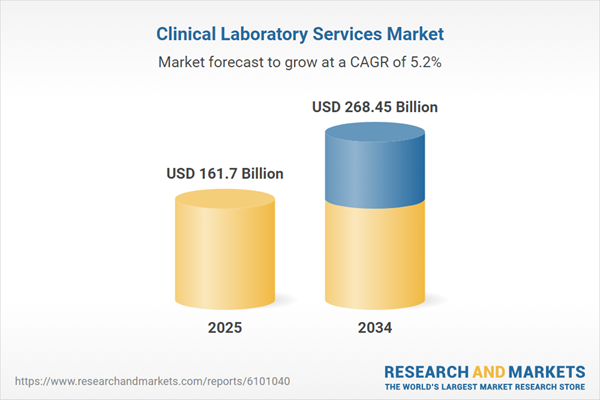
Clinical Laboratory Services Market Overview
Clinical laboratory services refer to diagnostic testing and analysis conducted on clinical specimens, such as blood, urine, or tissue, to provide information essential for disease prevention, diagnosis, and treatment. These services support healthcare professionals in making informed medical decisions and monitoring patient health. They encompass a wide range of disciplines, including hematology, microbiology, clinical chemistry, immunology, and molecular biology. Clinical laboratories play a crucial role in detecting infections, monitoring chronic conditions, and managing therapeutic interventions, thereby contributing significantly to improving patient outcomes and enhancing the overall efficiency of healthcare systems.Clinical Laboratory Services Market Growth Drivers
Workflow Digitization to Enhance Market Value in the Forecast Period
The increasing demand for digital transformation, coupled with the growing need for efficiency in clinical trials, is driving the adoption of automated laboratory solutions. For instance, in March 2025, IQVIA Laboratories introduced the Site Lab Navigator, an advanced suite of tools aimed at digitising and streamlining lab workflows for trial sponsors and investigator sites. Central to this solution is an innovative e-Requisition system that replaces manual paperwork with electronic processes, reducing administrative burden, human error, and improving compliance. This advancement is poised to accelerate market development by enhancing operational efficiency, enabling quicker trial execution, and fostering higher-quality data generation in clinical laboratory services.Investments in Companion Diagnostics to Boost Clinical Laboratory Services Market Demand
Rising investments in precision medicine and the need for regulatory-compliant laboratory services are reinforcing the value of companion diagnostics in clinical research. For instance, in September 2024, Agilent Technologies launched the Biopharma CDx Services Lab (BCSL) in California after securing CLIA registration, enabling it to meet stringent standards for human specimen testing. The lab offers end-to-end support from early clinical phases to FDA approvals, integrating advanced biomarker assessment technologies. This development is expected to fuel market growth by enhancing reliability, operational efficiency, and trust in laboratory outputs, thereby attracting more biopharma clients and strengthening the market presence of high-quality, regulatory-aligned service providers.Clinical Laboratory Services Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Digitalization Of Lab Services to Accelerate Market Growth
Digital transformation and workflow automation are becoming pivotal in modernising clinical laboratory services. The integration of cloud platforms, e-requisitions, and centralised data handling helps streamline operations and reduce manual errors. For instance, in June 2024, Labcorp introduced Labcorp Global Trial Connect, a central laboratory solution suite designed to enhance trial speed and efficiency at investigator sites. This development improves site performance and supports high-throughput testing needs. The trend of integrating digital tools in lab operations is poised to drive market expansion by enabling faster, more accurate, and cost-effective services, especially in decentralised and remote trial setups.Expansion Of Infectious Disease Diagnostics to Boost Clinical Laboratory Services Market Demand
The growing burden of infectious diseases, coupled with demand for rapid and accurate diagnostics, is pushing clinical laboratories to adopt advanced molecular tools. For instance, in October 2024, Delve Bio and Broad Clinical Labs announced a partnership to expand access to metagenomic next-generation sequencing (mNGS) for neurological infections. This collaboration strengthens the role of genomic diagnostics in clinical settings, offering precision diagnostics for complex pathogens. As mNGS becomes more accessible, it elevates the diagnostic capabilities of laboratories, reinforcing their market relevance. This trend will be instrumental in market growth, especially in infectious disease surveillance and precision medicine applications.Advanced Diagnostic Capabilities to Impact Clinical Laboratory Services Market Size Positively
In January 2024, HORIBA Medical introduced the HELO 2.0 high-throughput automated hematology platform, reflecting a growing trend in the clinical laboratory services market towards modular, scalable, and customer-driven diagnostic solutions. With CE-IVDR approval and pending US FDA clearance, this innovation demonstrates the industry's focus on regulatory compliance and global market expansion. Designed to meet the evolving needs of mid to large-scale laboratories, HELO 2.0 signifies the increasing demand for automation, flexibility, and efficiency in haematology testing. This launch underscores a broader market shift toward integrated, high-performance platforms that support improved workflow and enhanced diagnostic accuracy.Inclination Towards Outsourcing is a Another Notable Clinical Laboratory Services Market Trend
Pharmaceutical and biotechnology firms are increasingly outsourcing laboratory services to central labs for cost efficiency, scalability, and regulatory compliance. This trend is growing due to rising trial complexities and the need for standardised testing across multiple geographies. Outsourced labs offer specialised expertise, automation capabilities, and streamlined logistics, enabling faster clinical trial execution and drug development. Additionally, the demand for real-time data access and integrated analytics platforms has strengthened reliance on contract laboratory partners. This outsourcing momentum is expected to support market development by expanding global testing networks, boosting service portfolios, and enhancing trial efficiency for sponsors and contract research organisations.Clinical Laboratory Services Market Segmentation
Clinical Laboratory Services Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Test Type
- Clinical Chemistry Testing
- Routine Chemistry Testing
- Endocrinology Chemistry Testing
- Therapeutic Drug Monitoring (TDM) Testing
- Specialized Chemistry Testing
- Other Clinical Chemistry Testing
- Microbiology Testing
- Infectious Disease Testing
- Transplant Diagnostic Testing
- Other Microbiology Testing
- Hematology Testing
- Routine Hematology Testing
- Coagulation Testing
- Specialized Hematology Testing
- Immunology Testing
- Cytology Testing
- Genetic Testing
- Drugs of Abuse Testing
- Others
Market Breakup by Provider
- Standalone Laboratories
- Hospital-Based Laboratories
- Clinic-Based Laboratories
Market Breakup by Application
- Toxicology Testing Services
- Cell and Gene Therapy Related Services
- Preclinical and Clinical Trial Related Services
- Bioanalytical and Lab Chemistry Services
- Drug Discovery and Development Related Services
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Clinical Laboratory Services Market Share
Clinical Chemistry Testing to Lead the Segmentation by Test Type
Clinical chemistry testing is projected to hold the largest market share due to its broad applicability across routine health assessments and chronic disease monitoring. Increasing incidences of diabetes, cardiovascular disorders, and hormonal imbalances drive demand for routine and specialised chemistry testing. Innovations in biomarker identification and point-of-care diagnostics enhance test accuracy and speed, making them indispensable across healthcare settings. Additionally, the rising trend of personalised medicine and preventive healthcare supports further integration of chemistry panels in routine diagnostics. This segment’s versatility, affordability, and adaptability position it as a core driver of growth in the clinical laboratory services market during the forecast period.Standalone Laboratories to Hold a Significant Clinical Laboratory Tests Market Share by Provider
Standalone laboratories are expected to dominate the provider segment owing to their advanced infrastructure, high testing volumes, and operational flexibility. These facilities often house specialised equipment and trained personnel, enabling them to deliver a broad spectrum of high-quality tests at faster turnaround times. With growing demand for outpatient diagnostics and decentralised healthcare, standalone labs are expanding their footprint through partnerships and digital platforms. Moreover, their cost efficiency and scalability appeal to both public and private healthcare providers. These factors collectively make standalone laboratories a pivotal growth catalyst in the clinical laboratory services market in the years ahead.Drug Discovery and Development Services to Lead the Clinical Laboratory Tests Market Segmentation by Application
Drug discovery and development services are poised to lead the application segment, supported by increasing pharmaceutical R&D investment and a surge in novel drug pipelines. These services are critical for validating drug efficacy and safety across preclinical and clinical trial stages. Clinical laboratories offer essential bioanalytical and biomarker testing that accelerates the decision-making process in drug development. The rise of precision medicine, biologics, and gene therapies further elevates the need for sophisticated lab capabilities. As regulatory demands intensify, the reliance on laboratory partners for accurate and timely data continues to grow, positioning this segment at the forefront of market expansion.
Clinical Laboratory Services Market Analysis by Region
North America is likely to hold the largest market share, primarily driven by the region's strong healthcare infrastructure, high volume of diagnostic testing, and widespread adoption of advanced laboratory technologies. The United States, in particular, benefits from a robust insurance system, significant investment in personalised medicine, and a high burden of chronic diseases that necessitate routine and specialised testing. For instance, in February 2025, CytoChip received US Food and Drug Administration 510(k) clearance and a CLIA waiver for its complete blood count testing system, enhancing point-of-care diagnostics and reinforcing the region's leadership in laboratory innovation. Europe is also seeing notable growth, especially in Germany and the UK, due to expanding molecular diagnostics and home testing services. Meanwhile, Asia-Pacific is gaining momentum with increasing healthcare access, particularly in China and India, though limited reimbursement frameworks persist. Latin America and the Middle East & Africa show moderate growth, constrained by resource availability and infrastructure limitations.Leading Players in the Clinical Laboratory Services Market
The key features of the market report comprise patent analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Quest Diagnostics Incorporated
Founded in 1967 and headquartered in Secaucus, New Jersey, Quest Diagnostics Incorporated is a leading provider of diagnostic information services. The company specialises in clinical laboratory testing, anatomic pathology, and gene-based and esoteric testing. Quest operates a vast network of laboratories and patient service centres across the United States and internationally. Its services support physicians, hospitals, insurers, and pharmaceutical companies by offering accurate test results, wellness insights, and diagnostics innovations. Quest’s extensive portfolio and ongoing investment in digital health solutions position it strongly in the evolving clinical laboratory services market.Labcorp
Established in 1978 and based in Burlington, North Carolina, Labcorp is a global life sciences company providing comprehensive clinical laboratory and end-to-end drug development services. It operates one of the largest networks of central laboratories and specialises in routine and specialised testing, genomics, and clinical trials. Labcorp supports healthcare providers and pharmaceutical firms with services that span diagnostics, drug development, and companion diagnostics. Its strategic acquisitions and focus on precision medicine enhance its capabilities, ensuring its dominant role in clinical laboratory services worldwide.Davita Healthcare Partners
Headquartered in Denver, Colorado, and established in 1999, DaVita Healthcare Partners is renowned for its kidney care and dialysis services. Through its division, DaVita Labs, the company provides high-quality clinical laboratory services tailored for renal patients. It operates a high-volume laboratory focused on delivering fast, accurate diagnostic support to nephrologists. With a strong commitment to innovation and patient-centric care, DaVita leverages technology and standardised protocols to optimise lab efficiency and outcomes, thereby strengthening its position in the clinical laboratory services market, especially within nephrology-focused diagnostics.Spectra Laboratories
A subsidiary of Fresenius Medical Care, Spectra Laboratories was founded in 1982 and is headquartered in Rockleigh, New Jersey. It specialises in laboratory services for dialysis patients, offering testing in areas such as microbiology, haematology, and immunology. Spectra operates two major full-service laboratories in the U.S. and is known for its rapid turnaround, high-volume testing, and dialysis-specific diagnostic solutions. Its commitment to renal patient care and extensive logistics network make it a trusted partner for dialysis providers, positioning Spectra as a key contributor to the clinical laboratory services market’s growth.Other key players in the market include Eurofins Scientific, Unilabs, Synlab International, Bio-Reference Laboratories, ACM Medical Laboratory, and Lifelabs Medical Laboratories.
Key Questions Answered in the Clinical Laboratory Services Market
- What was the global clinical laboratory services market value in 2024?
- What is the global clinical laboratory services market forecast outlook for 2025-2034?
- What is market segmentation based on test type?
- What is market segmentation based on the provider?
- What is market segmentation based on application?
- What is market segmentation based on end user?
- What are the major factors aiding the global clinical laboratory services market demand?
- How has the market performed so far, and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major global clinical laboratory services market trends?
- Which type will lead the market segment?
- Which provider will lead the market segment?
- Which application will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the global clinical laboratory services market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Quest Diagnostics Incorporated
- Labcorp
- Davita Healthcare Partners
- Spectra Laboratories
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 161.7 Billion |
| Forecasted Market Value ( USD | $ 268.45 Billion |
| Compound Annual Growth Rate | 5.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |