Achondroplasia Market Overview
Achondroplasia is a genetic disorder causing short stature and disproportionately short limbs, due to abnormal bone growth. It is the most common form of dwarfism, caused by a mutation in the FGFR3 gene. Symptoms include a larger head, limited joint mobility, and potential complications affecting the spine and respiratory system.Achondroplasia Market Growth Drivers
FDA Approvals Drive Market Growth
Increasing investment in genetic disorder therapies and rising demand for targeted treatments are key drivers in the achondroplasia market. In September 2024, BridgeBio Pharma announced that its oral drug infigratinib, being developed for children with achondroplasia, received a Breakthrough Therapy Designation from the FDA. This designation aims to fast-track the drug’s development due to its potential to substantially improve clinical outcomes over existing therapies. This development is poised to significantly accelerate the market’s growth by fostering innovation and expediting product availability, expanding treatment options for achondroplasia patients.Surge in Clinical Trial Initiatives to Meet Rising Achondroplasia Market Demand
The growing focus on rare genetic diseases and advancements in targeted therapies are major drivers for the achondroplasia market. In October 2024, Tyra Biosciences announced that the FDA cleared its Investigational New Drug (IND) application for TYRA-300, an oral FGFR3-selective inhibitor for children with achondroplasia. The clearance allows the company to proceed with a Phase 2 trial. This promising first-in-class treatment could provide a safer and more effective option for achondroplasia patients, potentially transforming the treatment landscape and fostering market expansion in the forecast period.Achondroplasia Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Breakthrough Therapy Designation Boosts Market Growth for Rare Diseases
The growing emphasis on rare diseases and advancements in gene therapies are key market drivers. For instance, in September 2024, BridgeBio Pharma received FDA breakthrough therapy designation for its infigratinib, an oral treatment for achondroplasia. The designation was based on positive data from the PROPEL 2 phase 2 trial. This designation accelerates development, potentially improving treatment options for children with achondroplasia. The news is expected to drive market expansion, fostering innovation and enhancing patient access to effective therapies in the coming years.Collaboration Between Biopharmaceutical Companies Drives Achondroplasia Market Growth
Strategic collaborations between biopharmaceutical companies are becoming a prominent trend in the achondroplasia market. Partnerships between companies like BioMarin Pharmaceutical and QED Therapeutics are accelerating the development of targeted therapies for achondroplasia. For instance, in February 2024, BridgeBio Pharma and Kyowa Kirin announced a partnership in which BridgeBio’s affiliate, QED Therapeutics, granted Kyowa Kirin an exclusive license to develop and commercialise infigratinib (an oral FGFR3 inhibitor) for achondroplasia and other skeletal dysplasias in Japan. BridgeBio is likely to receive USD 100 million upfront payments, royalties, and milestone payments.Advancements in Small Molecule Inhibitors Impacts the Achondroplasia Market Size Positively
The development of small molecule inhibitors targeting the FGFR3 pathway is a growing trend in the achondroplasia market. These inhibitors aim to directly address the root cause of the condition by modifying the abnormal signalling that restricts bone growth. As clinical trials progress, these therapies offer the potential to significantly alter the treatment landscape, providing an oral, targeted approach to managing achondroplasia. This could reduce dependency on growth hormone therapy and provide a more effective, convenient option for patients.Growing Focus on Early Diagnosis and Treatment for Achondroplasia
The market is witnessing an increasing focus on early diagnosis and intervention. Early detection, often through genetic testing, enables timely treatment, which can improve outcomes and quality of life for affected individuals. This trend is pushing for broader adoption of genetic screening programs and the development of diagnostic tools that can identify achondroplasia in newborns. As awareness of the condition grows, early-stage treatments and preventative strategies will contribute to better market penetration and higher demand for therapies aimed at younger patient populations.Achondroplasia Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Treatment
- Growth Hormone Therapy
- Surgery
- Supportive Therapy
- Others
Market Breakup by Route of Administration
- Oral
- Parenteral
- Others
Market Breakup by End User
- Hospitals
- Home Care Settings
- Specialty Centers
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Achondroplasia Market Share
The growth hormone therapy is likely to hold a significant market share based on treatment. This is primarily due to its widespread use in children with achondroplasia to promote growth and improve height. Growth hormone therapy is considered the most effective non-invasive treatment to stimulate bone growth, especially when administered early in a child’s development. As per the analysis by Expert Market Research, the global human growth hormone market is anticipated to grow at a CAGR of 8.5% during the forecast period of 2025-2034. The therapy’s ability to provide significant improvements in stature, alongside relatively well-established treatment protocols, drives its dominance. Additionally, ongoing research into optimising growth hormone formulations and their long-term benefits continues to fuel its market leadership.Achondroplasia Market Analysis by Region
The United States holds the largest market share in the market, driven by its strong healthcare infrastructure and advanced research capabilities. The region leads in genetic research and clinical trials for rare diseases, with a high concentration of pharmaceutical companies focusing on rare genetic disorders like achondroplasia. Moreover, the United States benefits from robust reimbursement systems and significant government funding for rare disease therapies, enabling faster access to new treatments and therapies. The country's focus on personalised medicine and gene therapy innovation further fuels its market dominance.Leading Players in the Achondroplasia Market
The key features of the market report comprise patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:BioMarin Pharmaceutical Inc
BioMarin Pharmaceutical Inc., founded in 1997 and headquartered in San Rafael, California, USA, is a global biopharmaceutical company specialising in treatments for rare genetic disorders. In the achondroplasia market, BioMarin is developing vosoritide, a first-in-class peptide therapy aimed at stimulating bone growth in children with achondroplasia. For instance, in March 2023, the FDA accepted the supplemental New Drug Application (sNDA) for VOXZOGO (vosoritide) to expand its use in the U.S. to treat children under 5 with achondroplasia, a common form of short stature.QED Therapeutics
QED Therapeutics, established in 2016 and headquartered in San Francisco, California, USA, is a biopharmaceutical company focused on developing therapies for rare genetic disorders. In the achondroplasia market, QED is focused on the development of small molecule inhibitors targeting the FGFR3 pathway, which is crucial in the development of achondroplasia. Their research aims to develop a treatment that can address the underlying cause of the condition, potentially offering a disease-modifying therapy for affected individuals.Ascendis Pharma A/S
Ascendis Pharma A/S, founded in 2006 and headquartered in Hellerup, Denmark, is a biotechnology company developing therapies for rare diseases. Ascendis Pharma is advancing TransCon CNP, a novel treatment for achondroplasia. This innovative therapy is designed to provide continuous activity of C-type natriuretic peptide (CNP), aiming to improve bone growth in children with achondroplasia. Ascendis Pharma’s approach offers a promising treatment option that could enhance growth rates and quality of life for affected patients.Pfizer Inc
Pfizer Inc., founded in 1849 and headquartered in New York City, USA, is a global leader in pharmaceuticals and vaccines. In the achondroplasia market, Pfizer is collaborating with other biopharma companies on the development of therapies for rare diseases. Pfizer’s research is focused on developing novel treatments that target the genetic and molecular pathways involved in conditions like achondroplasia, with a focus on advancing innovative therapies to address unmet needs in pediatric and adult populations affected by growth disorders. For instance, in May 2019, Pfizer announced its acquisition of Therachon Holding AG for USD 340 million upfront, with up to USD 470 million in milestone payments. Therachon’s assets include treatments for achondroplasia and short bowel syndrome, conditions with significant unmet medical needs.Other key players in the market include Ribomic Inc., Astellas Pharma Inc., Sanofi S.A., and Novo Nordisk A/S.
Key Questions Answered in the Achondroplasia Market
- What was the global achondroplasia market value in 2024?
- What is the chondroplasia market forecast outlook for 2025-2034?
- What is the market breakup based on the treatment?
- What is the market breakup based on the route of administration?
- What is the market breakup based on the end user?
- What are the major factors aiding achondroplasia market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major achondroplasia market trends?
- Which treatment will lead the market segment?
- Which route of administration will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the achondroplasia market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- BioMarin Pharmaceutical Inc.
- QED Therapeutics
- Ascendis Pharma A/S
- Pfizer Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
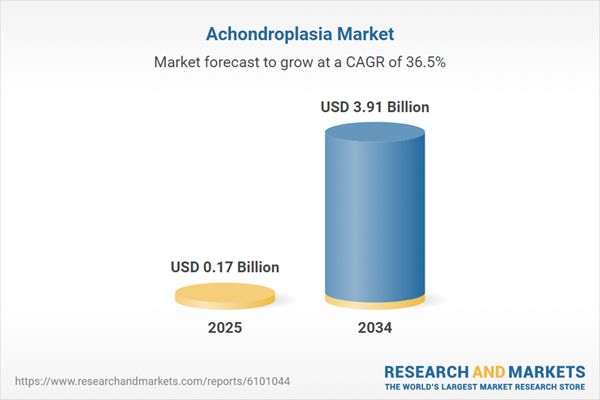
| Estimated Market Value ( USD | $ 0.17 Billion |
| Forecasted Market Value ( USD | $ 3.91 Billion |
| Compound Annual Growth Rate | 36.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |