Trichomoniasis Market Overview
Trichomoniasis is a common sexually transmitted infection (STI) caused by the parasite Trichomonas vaginalis. It affects both men and women, though symptoms are more prominent in women, including vaginal discharge, itching, and discomfort. Men may experience urethral discharge or irritation. Early detection and treatment with antibiotics are crucial to prevent complications and transmission.Trichomoniasis Market Growth Drivers
Global Health Strategies Set to Drive Trichomoniasis Market Expansion
Rising global awareness of sexually transmitted infections (STIs) and enhanced government healthcare initiatives are key drivers of growth in the market. According to the World Health Organization’s November 2024 fact sheet, approximately 156 million new cases of Trichomonas vaginalis infection occurred in 2020 among individuals aged 15-49. The WHO’s 2022-2030 Global Health Sector Strategy aims to reduce new cases of trichomoniasis by 50% by 2030. This ambitious target underscores the growing need for effective diagnostics, treatments, and preventive measures, thus fostering market expansion during the forecast period. Increased government investments and policy shifts will likely boost innovation in both therapeutic and diagnostic solutions, further driving market growth.New Treatment Approvals to Boost Trichomoniasis Market Value
The growing focus on improving treatment options and approval of innovative therapies are key market drivers for the trichomoniasis sector. For instance, in February 2022, Lupin Pharmaceuticals announced the U.S. FDA’s approval of a supplemental New Drug Application (sNDA) for SOLOSEC (secnidazole), expanding its indication to include both bacterial vaginosis (BV) and trichomoniasis in patients aged 12 and older. SOLOSEC becomes the first and only single-dose oral antimicrobial agent approved for treating both conditions. This approval positions the product as a significant player in the trichomoniasis treatment market. The simplified, single-dose regimen is likely to improve patient compliance, potentially increasing the uptake of trichomoniasis treatments, and further driving market expansion.Trichomoniasis Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Increasing Investment in Trichomoniasis Research and Development Driving Market Growth
The trichomoniasis treatment market is witnessing a surge in research and development activities, driven by pharmaceutical companies focused on developing more effective and targeted therapies. Advancements in drug formulations and the introduction of combination therapies aim to improve cure rates and reduce recurrence. This trend is expected to drive market growth and enhance treatment options, increasing patient satisfaction and adherence.Adoption of Rapid Diagnostic Tools Contributing to Trichomoniasis Market Demand
Point-of-care diagnostic tools for trichomoniasis are gaining popularity, especially in resource-limited settings. Rapid, easy-to-use, and cost-effective tests are becoming a key market trend, enabling faster detection and treatment. With increased accessibility, healthcare providers can diagnose infections promptly, leading to quicker interventions, reduced transmission, and improved patient outcomes. This trend is contributing significantly to market development and expansion.Surge in FDA Approvals to Accelerate Trichomoniasis Market Value Positively
The approval of new trichomoniasis treatments by the U.S. Food and Drug Administration (FDA) is set to enhance market dynamics. These approvals are expected to improve treatment options and provide more targeted therapies, addressing antibiotic resistance and offering faster recovery. For instance, in September 2023, the FDA approved Likmez (metronidazole), a ready-to-use liquid suspension formulation developed by Appili Therapeutics Inc. and Saptalis Pharmaceuticals, LLC, for the treatment of bacterial infections, trichomoniasis, and amebiasis. FDA-backed innovations will likely inspire greater investments in the research and development of next-generation treatments, further supporting market growth.Preventative Healthcare Strategies Enhancing Long-Term Trichomoniasis Market Size
Preventative healthcare strategies, such as pre-exposure prophylaxis (PrEP) for high-risk populations, are becoming more common in the fight against sexually transmitted diseases like trichomoniasis. Healthcare providers are emphasizing prevention, with screening, vaccination, and behavioural interventions playing crucial roles. This proactive approach is expected to reduce infection rates and enhance long-term market value by decreasing overall treatment demand.Trichomoniasis Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Drug Type
- Metronidazole
- Tinidazole
- Others
Market Breakup by Dosage Form
- Oral
- Intravenous
- Suppository
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Homecare Settings
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Trichomoniasis Market Share
Market Segmentation Based on Dosage Form to Witness Significant Growth
The oral dosage form is predicted to lead the market due to its convenience, ease of administration, and high patient compliance. Oral medications, such as metronidazole and tinidazole, are preferred as they can be easily taken at home, eliminating the need for medical supervision. This segment's dominance is driven by widespread availability, cost-effectiveness, and patient preference for non-invasive treatment options. Oral formulations also enable mass distribution in both developed and emerging markets, further expanding their market share.Trichomoniasis Market Analysis by Region
The United States leads due to its advanced healthcare infrastructure, high awareness of sexually transmitted infections (STIs), and strong pharmaceutical sector. The presence of major global pharmaceutical companies, extensive research and development, and widespread healthcare access drive market dominance. Additionally, significant investments in STI diagnostics and treatments, coupled with robust public health campaigns, contribute to the U.S. market’s leadership, making it the largest and most developed segment in the global trichomoniasis market.Leading Players in the Trichomoniasis Market
The key features of the market report comprise patent analysis, clinical trials analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Pfizer Inc
Headquartered in New York, USA, Pfizer was established in 1849 and is a global leader in the pharmaceutical industry. The company’s portfolio includes a wide range of treatments for infectious diseases, including trichomoniasis. Pfizer offers effective antimicrobial therapies, with a focus on developing novel treatments to combat STIs and improve patient outcomes.Lupin
Lupin, based in Mumbai, India, was founded in 1968. A prominent player in the global pharmaceutical market, Lupin’s portfolio includes generics, specialty medications, and active pharmaceutical ingredients (APIs). The company produces a range of antibiotics and treatments for infectious diseases, including trichomoniasis, leveraging its expertise in affordable, high-quality medications for global markets.Zydus Group
Zydus Group, headquartered in Ahmedabad, India, was established in 1952 and is a leading global pharmaceutical company. Its portfolio includes a variety of therapeutic segments, including antibiotics and treatments for infectious diseases. Zydus Group offers affordable generics for trichomoniasis, with a focus on improving access to essential medications across emerging markets.F. Hoffmann La Roche
F. Hoffman La Roche, founded in 1896 and headquartered in Basel, Switzerland, is a global leader in pharmaceuticals and diagnostics. Known for its innovative treatments, Roche’s portfolio spans oncology, infectious diseases, and diagnostics. The company’s contributions to the trichomoniasis market focus on research and developing diagnostic tools, alongside its range of antiviral therapies for STIs.Other key players in the market include Kesin Pharma and Sanofi.
Key Questions Answered in the Trichomoniasis Market
- What was the Trichomoniasis market value in 2024?
- What is the Trichomoniasis market forecast outlook for 2025-2034?
- What is the market breakup based on the drug type?
- What is the market breakup based on the dosage form?
- What is the market breakup based on the end user?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major Trichomoniasis market trends?
- Which drug type will lead the market segment?
- Which dosage form will lead the market segment?
- Which end user will lead the market segment?
- Who are the key players involved in the Trichomoniasis market?
- What is the patent landscape of the market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Lupin
- Zydus Group
- F. Hoffmann La Roche
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
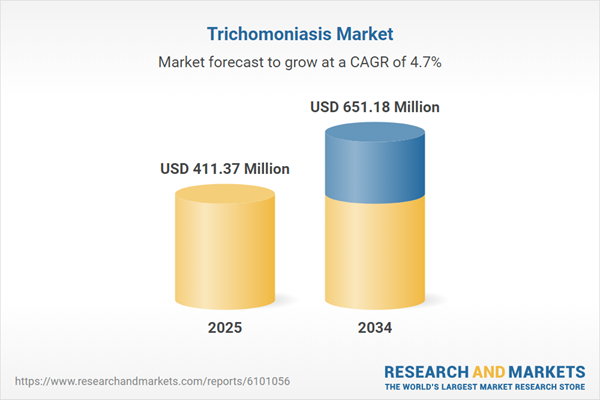
| Estimated Market Value ( USD | $ 411.37 Million |
| Forecasted Market Value ( USD | $ 651.18 Million |
| Compound Annual Growth Rate | 4.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |