Radioimmunoassay Market Report by Product (Analyzers, Reagents and Kits), Application (Research, Clinical Diagnostics), End User (Hospitals, Clinical Diagnostic Laboratories, Pharmaceutical Industries, Other End Users), Countries and Company Analysis, 2025-2033.
Global Radioimmunoassay Market Overview
Radioimmunoassay (RIA) is a sensitive laboratory method for the determination of trace levels of hormones, drugs, or other compounds in body fluids. RIA is based on antigen-antibody reaction in which a measured amount of radioactively labeled compound is mixed with the unlabeled compound from the sample for a fixed number of antibody binding sites. The level of detected radioactivity is inversely related to the concentration of the compound in the sample.RIA has transformed medical diagnosis from its inception in the 1960s, particularly in endocrinology, where it permitted accurate quantitation of hormones including insulin, thyroid hormones, and cortisol. Even though it has been increasingly displaced by non-radioactive counterparts such as ELISA on account of safety and handling issues, RIA is still widely used in certain clinical and research applications on account of its high sensitivity and specificity. It remains to be extensively applied in specialized laboratories for detecting drugs, hormones, and even tumor markers, keeping its use relevant in medical and pharmaceutical research.
Growth Drivers in the Radioimmunoassay Market
Increased Demand for Early and Precise Diagnosis of Diseases
The growing incidence of infectious and chronic conditions has boosted the demand for precise diagnostic products. Radioimmunoassay (RIA) is extremely useful in the detection of low doses of drugs, hormones, and antigens, and is therefore crucial in the diagnosis of thyroid conditions, certain malignancies, and endocrine disorders. As medical systems are prioritizing preventive therapy, RIA is becoming more commonly utilized by hospitals and clinical laboratories to enhance the health outcomes of patients by facilitating faster diagnosis. In March 2023, the National Foundation of Infectious Diseases reported that 1.2 million U.S. citizens were infected with HIV, with 13% not knowing they had the disease, and there are approximately 30,000+ new cases every year. In addition, Global HIV & AIDS statistics Fact sheet Global HIV statistics 39.9 million [36.1 million-44.6 million] people in the world were living with HIV in 2023.Technological Improvement in RIA Kits and Automation
Technology is the key driving force in the radioimmunoassay market. New-generation RIA kits are more user-friendly, precise, and automated, which minimizes human error and maximizes workflow efficiency. Technological advancements, for example, robotic liquid handling systems, enable high-throughput testing with reduced human intervention. Such enhancements make RIA accessible to more labs, including those in constrained environments. Additionally, advancements in radioisotope safety and handling procedures are making the technology safer and more environmentally friendly, which spurs increased use in both industrialized and developing healthcare systems. Another key driver of the growth of the radioimmunoassay market is the very high sensitivity and specificity of RIA methods in contrast to most other immunoassays.Growing Applications in Pharmaceutical and Biomedical Research
RIA is an important component in drug development, pharmacokinetics, and clinical trials. Pharmaceutical firms apply it to quantify drug levels and biological effects for the assurance of efficacy and safety. Its sensitivity enables scientists to track low-dose responses and perform thorough biomarker studies. With heightened investment in life science research, especially in the developing countries, demand for RIA in academic and commercial R&D is on the increase. Moreover, growth of personalized medicine and targeted therapies has amplified the requirement of accurate biomolecular measurement tools, further fueling RIA use in clinical as well as non-clinical research settings.Challenges in the Radioimmunoassay Market
Strict Regulatory Controls and Radioactive Waste Management
One of the main hurdles for the RIA market is regulatory concern over the application and disposal of radioactive sources. Because of the inherent danger of radioisotopes, laboratories are required to meet stringent safety requirements and environmental regulations, which can be time-consuming and expensive. Overregulation could hold up product approvals or restrict use in some areas. Additionally, appropriate radioactive waste disposal involves trained staff and dedicated facilities, which might not be present in every healthcare or research environment. Such challenges will discourage new entrants and restrict the growth of RIA technologies within less-developed economies.Competition from Non-Radioactive Alternatives
The availability of non-radioactive immunoassay methods like ELISA (enzyme-linked immunosorbent assay) and chemiluminescent immunoassays threatens the RIA market extensively. These alternatives provide comparable sensitivity without the hassles of radioactive handling, storage, or waste disposal. With improving technology, these newer techniques are becoming more cost-efficient, gaining general acceptance, and easier to utilize in clinical practice. Laboratories and hospitals tend to favor non-radioactive techniques because of their lower risk for safety and easier compliance. This transition in preference is increasingly diminishing demand for conventional RIA, particularly in countries with harsh regulatory regimes.Reagents and Kits Radioimmunoassay Market
Reagents and kits constitute the core of the RIA process, supplying the chemicals, antibodies, and radioisotopes needed for precise testing. This market is experiencing consistent growth based on the rising number of diagnostic and research applications that demand precise measurement of hormone and biomarker quantification. With the expansion of healthcare systems across the world, the need for ready-to-use, high-sensitivity RIA kits is on the increase. Firms are investing in creating safer, longer shelf-life kits with improved automation compatibility. The increased requirement for reliable diagnostics in the fields of endocrinology, oncology, and infectious diseases continues to drive growth in this segment, especially in clinical laboratories and academic research environments.Radioimmunoassay Clinical Diagnostics Market
The clinical diagnostics market leads the RIA market because of its essential role in detecting and controlling disease. RIA finds extensive application in hormone level measurements, cancer markers, allergy diagnosis, and infectious disease monitoring. Due to its sensitivity, it cannot be replaced for early detection, especially for thyroid functions and reproductive hormone measurements. With the rising incidence of lifestyle and chronic diseases across the globe, so rises the demand for sophisticated diagnostics. RIA's reliability and accuracy are a boon to clinical facilities, enabling doctors to make prompt, informed decisions. Despite increased competition from newer technology, RIA continues to be relevant based on its tried-and-tested accuracy and specificity.Radioimmunoassay Clinical Diagnostic Laboratories Market
Clinical diagnostic laboratories are among the major users of RIA technologies, conducting a large number of tests for hospitals, clinics, and research institutions. These laboratories need regular, economical, and scalable tests - a requirement that RIA fulfills effectively. Several laboratories are moving towards automated systems for greater efficiency, and RIA kits are adapting to such requirements. Centralized lab services, growth of, and outsourcing diagnostics globally also benefit from growth in this sector. In addition, specialized diagnostic laboratories utilize RIA for drug testing and hormone panels because it offers unparalleled sensitivity in the detection of low concentrations. Worldwide, there were approximately an estimated 1.52 million people under the age of 20 years living with type 1 diabetes in 2022 and 8.4 million people with type 1 diabetes in 2021, with an expected growth to 13.5-17.4 million by 2040.United States Radioimmunoassay Market
The United States dominates the radioimmunoassay (RIA) market owing to rising awareness of long-term ailments such as cancer and thyroid diseases, which demand proper monitoring. A competent workforce and close academic partnership fuel diagnostic techniques advancements. The market is consistently expanding with assistance from healthcare innovation and clinical requirements. Domestic production of Iridium-192 (Ir-192), a key radioisotope for industrial and medical use, has been announced by Oak Ridge National Laboratory in February 2024, minimizing U.S. reliance on foreign supplies. This will enhance RIA firms' assay technologies, facilitating consistency in research and production. In 2023, the United States will see 1,958,310 new cancer diagnoses and 609,820 cancer deaths, as estimated by the American Cancer Society.United Kingdom Radioimmunoassay Market
The UK radioimmunoassay market is expanding based on robust clinical study and healthcare infrastructure investment. The NHS encourages sophisticated diagnostic practices in primary and secondary care. There's increasing demand for precise hormone and disease marker testing, particularly in oncology and reproductive health. The successful life sciences industry consists of both local and foreign businesses engaged in manufacturing and distributing radioimmunoassay kits and systems. In November 2024, The BBC cited a dire shortage of medical radioactive isotopes in the UK because of a reactor shutdown in the Netherlands, resulting in cancer diagnostic delays and possibly increased fatality rates. Experts are calling for production to take place locally, with Project Arthur suggesting a €400 million medical lab at the former nuclear complex in Trawsfynydd, Wales, to manufacture vital radioactive materials.China Radioimmunoassay Market
The market for radioimmunoassays in China is growing very quickly because of the fast-growing conditions of chronic diseases such as thyroid disease, cancer, and diabetes, which increase the need for accurate laboratory tests. China's domestic biotech and diagnostic manufacturing industry is coming of age, manufacturing quality assay kits to meet local specifications. China's emphasis on accuracy in healthcare supports the application of radioimmunoassay for clinical diagnostics and research. Japan's aging population similarly creates demand for age-related disease diagnostic tests, and its well-equipped labs support smooth incorporation of radioimmunoassay into standard workflows. July 2023, China's Fapon debuted its new chemiluminescence immunoassay system, Shine i8000/9000, at the 2023 AACC Scientific Meeting and Clinical Lab Expo.Brazil Radioimmunoassay Market
The Brazilian radioimmunoassay market is growing considerably. With its growing focus on laboratory services and its evolving public healthcare infrastructure, Brazil is becoming one of Latin America's leading markets for radioimmunoassay diagnostics. The country has been striving to expand access and reduce health disparities through the expansion of its diagnostic facilities both in the public and private sector. Credible testing methods such as radioimmunoassay are gaining increasing popularity in Brazil because of the country's large population base and growing rates of diseases such as thyroid abnormalities, viral diseases, and hormone-related issues.Saudi Arabia Radioimmunoassay Market
The Middle East and Africa (MEA) radioimmunoassay market is poised to grow as portable, affordable solutions are gaining acceptance in less developed regions and sophisticated systems being taken up in high-income nations. There is a growing focus on the detection of early disease, including HIV and thyroid conditions, backed by campaigns from health bodies and governments. The radioimmunoassay market in Saudi Arabia is booming with investments in research institutions and medical cities that create a hub for diagnostics innovation. The availability of funding and skilled personnel, who are technology aware, supports the introduction of new diagnosis platforms, thereby making the country a market leader in the region. September 2023, Grifols made a strategic alliance to boost its product range in the region, which is likely to consolidate its market standing.Market Segmentation
Disease Types
1. Seasonal ALLERGIC Conjunctivitis (SAC)2. Perennial Allergic Conjunctivitis (PAC)
Drugs Class
1. Antihistamines & Mast Cell Stabilizers2. Corticosteroids
3. Others
Distribution Channels
1. Hospital Pharmacies2. Online Pharmacies
3. Retail Pharmacies & Drug Stores
4. Others
Countries
North America
1. United States2. Canada
Europe
1. France2. Germany
3. Italy
4. Spain
5. United Kingdom
6. Belgium
7. Netherlands
8. Turkey
Asia Pacific
1. China2. Japan
3. India
4. Australia
5. South Korea
6. Thailand
7. Malaysia
8. Indonesia
9. New Zealand
Latin America
1. Brazil2. Mexico
3. Argentina
Middle East & Africa
1. South Africa2. Saudi Arabia
3. UAE
All the Key players have been covered from 5 Viewpoints:
- Overview
- Key Person
- Product Analysis
- Recent Development
- Revenue
Key Players Analysis
- Danaher (Beckman Coulter, Inc.)
- Berthold Technologies GmbH & Co. KG
- DIAsource ImmunoAssays SA
- BioCheck, Inc. (DRG International Inc.)
- IBL International
- Merck KGaA
- Abbexa
- Institute of Isotopes Co. Ltd.
- Marin Biologic Laboratories, Inc.
- Demeditec Diagnostics GmbH
Table of Contents
Companies Mentioned
- Danaher (Beckman Coulter, Inc.)
- Berthold Technologies GmbH & Co. KG
- DIAsource ImmunoAssays SA
- BioCheck, Inc. (DRG International Inc.)
- IBL International
- Merck KGaA
- Abbexa
- Institute of Isotopes Co. Ltd.
- Marin Biologic Laboratories, Inc.
- Demeditec Diagnostics GmbH
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | June 2025 |
| Forecast Period | 2024 - 2033 |
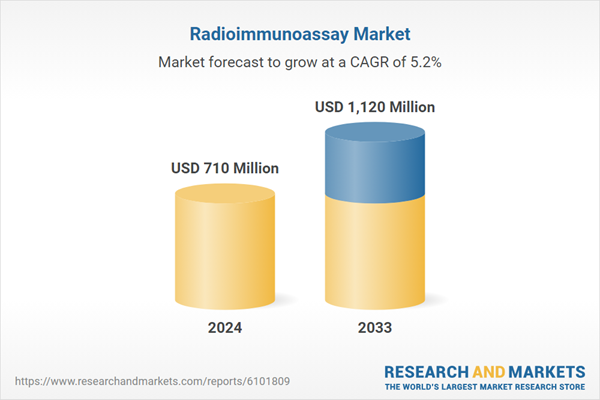
| Estimated Market Value ( USD | $ 710 Million |
| Forecasted Market Value ( USD | $ 1120 Million |
| Compound Annual Growth Rate | 5.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |