Nucleic Acid Amplification Testing Global Market Report by Type (Polymerase Chain Reaction (PCR) Tests, Isothermal Nucleic Acid Amplification Technology (INAAT) Tests, Ligase Chain Reaction (LCR) Tests), Application (Infectious Disease Testing, Oncology Testing, Others), End Use (Central & Reference Laboratories, Hospitals, Others), Countries and Company Analysis, 2025-2033.
Global Nucleic Acid Amplification Testing Industry Overview
The market is growing as a result of increased R&D expenditures for creating innovative biotechnology diagnostic methods and rising demand for sophisticated fast diagnostics. Additionally, the need for various nucleic acid amplification testing methods has been driven by the rising frequency of infectious illnesses worldwide and the requirement for highly effective testing options to effectively control disease outbreaks. The need for quick and precise diagnostic techniques, including nucleic acid amplification, to identify the SARS-CoV-2 virus has grown as a result of COVID-19. To satisfy the strong demand for quick and point-of-care testing, major market participants have created novel product offerings, such as tests based on isothermal nucleic acid amplification technology.For example, Abbott Laboratories' Abbott ID NOW COVID-19 test, which can provide positive findings in five minutes and negative results in thirteen, was granted Emergency Use Authorization (EUA) in March 2020. It is anticipated that these pandemic-driven strategic efforts would accelerate market expansion in the near future.
New opportunities for market expansion are being created by emerging infectious illnesses like the monkeypox virus. The introduction and regulatory approval of nucleic acid amplification test items for certain illnesses is proof of this. For example, Quest Diagnostics was granted the Emergency Use Authorization (EUA) by the U.S. FDA in September 2022 for their Quest Monkeypox PCR test, which was introduced in July 2022. Likewise, Todos Medical Ltd. commercially introduced their Monkey Pox PCR-based diagnostic in the United States in August 2022. Therefore, it is anticipated that these new infections would accelerate market expansion.
Key Factors Driving the Nucleic Acid Amplification Testing Market Growth
Shift toward point-of-care testing
By facilitating quick, on-site testing at dispersed locations like clinics, airports, and distant places, the move toward point-of-care testing is revolutionizing the diagnostic environment. The need for faster, more precise findings without the delays of central lab processing is what is driving this trend. Businesses like as Cepheid are developing small, easy-to-use systems.For example, Cepheid introduced a new proof of concept system in September 2024 that offers quick findings for a variety of diseases, including respiratory viruses, and aims to streamline molecular diagnostics in primary care and emergency situations.
The use of point-of-care NAAT in healthcare settings is expected to become increasingly important as technology develops, helping to reduce the spread of infectious illnesses and improve patient care.
Transition to high-throughput systems and automation
As labs look to increase productivity, reduce human error, and scale operations to meet high test numbers, there is a growing need for automation and high-throughput technologies in NAAT. By combining the procedures for sample preparation, amplification, and analysis, fully automated systems optimize workflows and provide quicker turnaround times and more reliable findings.To further increase throughput, businesses are making large investments in creating sophisticated robotic equipment and software that can handle several samples at once.
For example, Hamilton unveiled the Microlab STAR automated liquid handling system in February 2023. This system is intended to support high-throughput workflows, enhancing the accuracy and efficiency of sample processing in molecular diagnostics labs.
Developments in technology
The performance and accessibility of these diagnostic instruments are greatly improved by technological improvements, which are a major factor in the worldwide market's growth. Faster diagnostic and treatment decisions are now possible thanks to advancements in amplification techniques including loop-mediated isothermal amplification (LAMP) and fast polymerase chain reaction (PCR), which have significantly increased test speed and efficiency.As an illustration, Bio-Rad Laboratories introduced ddPCR (digital droplet PCR) technology in August 2023, which greatly increases sensitivity and precision in identifying low-abundance nucleic acids and advances the potential of molecular diagnostics. This technology was introduced in July 2024.
By enabling remote monitoring and real-time data sharing, this integration with digital health solutions - such as telehealth platforms and data analytics - further increases the usefulness of these tests and propels market expansion.
Challenges in the Nucleic Acid Amplification Testing Market
High Cost of Equipment and Tests
One major obstacle to wider implementation of nucleic acid amplification testing (NAAT) is its high cost, especially in low- and middle-income areas. Expensive equipment and specific reagents that must adhere to strict quality requirements are needed for advanced NAAT technologies. Furthermore, these systems frequently require highly skilled workers and a well-equipped laboratory setting to function. Smaller healthcare facilities and locations with limited resources may find these financial and infrastructure requirements onerous. Because of this, even though NAAT has a high sensitivity and specificity, it is still not widely available in many regions of the world. This expense barrier contributes to inequities in healthcare delivery and results by impeding prompt diagnosis and treatment in addition to affecting the use of certain tests.Complex Regulatory Landscape
It takes a lot of effort and resources to navigate the regulatory environment for nucleic acid amplification testing (NAAT). Strict standards for clinical validation, quality assurance, and performance testing are enforced by regulatory agencies, such as the FDA in the United States and the EMA in Europe. To prove accuracy, sensitivity, specificity, and repeatability under many circumstances, developers must submit copious amounts of data. These procedures frequently entail drawn-out clinical studies and thorough paperwork, which postpones market entrance and raises development costs dramatically. These requirements can be especially taxing on startups and smaller businesses. Global commercialization is further hampered by disparate foreign regulatory regulations. Public health results may be impacted by this disjointed and drawn-out approval procedure, which can impede innovation and limit patients' access to state-of-the-art diagnostic tools.Nucleic Acid Amplification Testing Market Overview by Regions
Regional differences in the NAAT market include North America's superior healthcare systems, Europe's emphasis on innovation and regulation, Asia-Pacific's explosive expansion, and emerging nations' access and infrastructure issues. The following provides a market overview by region:United States Nucleic Acid Amplification Testing Market
The market for nucleic acid amplification testing in the US is a crucial part of the country's diagnostic environment, which is distinguished by its sophisticated healthcare system and strong R&D environment. The diagnosis of viral illnesses, genetic abnormalities, and some types of cancer depends on NAAT approaches, such as polymerase chain reaction and isothermal amplification techniques. The rising incidence of infectious and chronic illnesses, the increased need for quick and precise diagnostics, and large expenditures in biotechnology and diagnostics are some of the reasons driving the market. Additionally, the United States enjoys robust reimbursement laws and a favorable regulatory environment, which support the broad implementation of NAAT across a range of healthcare settings, including as hospitals, diagnostic labs, and point-of-care facilities. This all-encompassing strategy highlights America's dominance in the worldwide NAAT business.United Kingdom Nucleic Acid Amplification Testing Market
The nucleic acid amplification testing (NAAT) market in the United Kingdom represents a sizeable portion of the larger European diagnostics business. Integrating NAAT for a range of uses, such as genetic screening and infectious illness detection, has been made possible in large part by the UK's sophisticated healthcare system, which is headed by the National Health Service (NHS). The COVID-19 pandemic significantly sped up the use of NAAT, demonstrating its effectiveness in quick and precise diagnosis. This change has led to further expenditures in molecular diagnostics, which has improved testing capacity and encouraged innovation. Additionally, the UK's dedication to biotechnology research and development guarantees that NAAT technologies will continue to advance. The UK continues to lead NAAT developments, striking a balance between innovation and regulatory supervision to uphold high diagnostic standards as the need for customized treatment and early illness identification increases.India Nucleic Acid Amplification Testing Market
The market for nucleic acid amplification testing (NAAT) in India is developing quickly due to rising healthcare expenditures and the rising prevalence of infectious disorders. Particularly since the pandemic, the nation has seen a sharp increase in testing due to the broad use of technologies like PCR and isothermal amplification techniques. Particularly in underprivileged areas, government programs like the National Tuberculosis Elimination Program have made it easier to include quick molecular tests like Truenat and CBNAAT into standard diagnostic procedures. Enhancing early illness diagnosis and expanding access to healthcare are the goals of these initiatives. The industry is expected to continue growing despite obstacles including high costs and inadequate infrastructure, thanks to continuous improvements in diagnostic technology and an emphasis on increasing testing capacities nationwide.United Arab Emirates Nucleic Acid Amplification Testing Market
The market for nucleic acid amplification testing (NAAT) in the United Arab Emirates (UAE) is expanding quickly due to improvements in molecular diagnostics and higher healthcare spending. The UAE's healthcare system relies heavily on NAAT technologies, such polymerase chain reaction (PCR) and isothermal amplification techniques, which make it easier to identify genetic and infectious illnesses. The introduction of these tools was further pushed by the COVID-19 pandemic, underscoring their significance in public health response and surveillance. The incorporation of NAAT into clinical practice has been strengthened by government programs and collaborations with foreign healthcare professionals. The UAE is a leader in the regional molecular diagnostics market because of its dedication to innovation and high-quality healthcare, even in the face of obstacles like exorbitant costs and the requirement for specialized training.Recent Developments in Nucleic Acid Amplification Testing Industry
- October 2024 - The first diagnostic test for mpox, a disease brought on by the monkeypox virus, was authorized by the World Health Organization (WHO). In light of the disease's recent recurrence, its approval is a critical step in strengthening international efforts to identify and treat it. The test's excellent accuracy and efficiency guarantee quicker diagnosis and more effective containment tactics during outbreaks.
Market Segmentations
Type
- Polymerase Chain Reaction (PCR) Tests
- Isothermal Nucleic Acid Amplification Technology (INAAT) Tests
- Ligase Chain Reaction (LCR) Tests
Application
- Infectious Disease Testing
- Oncology Testing
- Others
End Use
- Central & Reference Laboratories
- Hospitals
- Others
Regional Outlook
North America
- United States
- Canada
Europe
- France
- Germany
- Italy
- Spain
- United Kingdom
- Belgium
- Netherlands
- Turkey
Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Thailand
- Malaysia
- Indonesia
- New Zealand
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- Saudi Arabia
- United Arab Emirates
- South Africa
All the Key players have been covered
- Overview
- Key Persons
- Recent Development
- SWOT Analysis
- Revenue Analysis
Company Analysis:
- F. Hoffmann-La Roche Ltd
- Becton, Dickinson and Company
- Danaher Corporation
- Abbott Laboratories
- Illumina, Inc.
- Siemens Healthineers
- bioMérieux SA
- Novartis AG
- Bio-Rad Laboratories, Inc.
- Seegene Inc.
Table of Contents
Companies Mentioned
- F. Hoffmann-La Roche Ltd
- Becton, Dickinson and Company
- Danaher Corporation
- Abbott Laboratories
- Illumina, Inc.
- Siemens Healthineers
- bioMérieux SA
- Novartis AG
- Bio-Rad Laboratories, Inc.
- Seegene Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | June 2025 |
| Forecast Period | 2024 - 2033 |
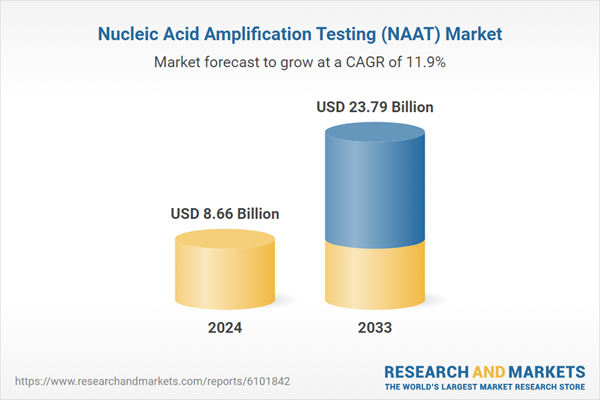
| Estimated Market Value ( USD | $ 8.66 Billion |
| Forecasted Market Value ( USD | $ 23.79 Billion |
| Compound Annual Growth Rate | 11.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |