STD Diagnostic Market Report by Product (Consumables, Instruments& Services, Software), Type (Chlamydia Testing, Gonorrhea Testing, Herpes Simplex Virus Testing, Human Papilloma Virus Testing, Human Immunodeficiency Virus Testing, Chancroid Testing, Others), Location of Testing (Laboratory Testing, Point of Care Testing), Countries and Company Analysis 2025-2033.
STD Diagnostic Market Overview
HIV, chlamydia, gonorrhea, syphilis, and HPV are some of the sexually transmitted diseases that can be detected by means of testing and processes referred to as STD diagnostics. Lab-based testing like blood tests, urine tests, vaginal swabs, and NAA tests (NAATs) are some of these tests. Home kits and rapid point-of-care tests have accelerated and made detection easier. Proper treatment, prevention of complications, and disease transmission reduction all rely on early and accurate diagnosis. Increasing numbers of individuals are being tested more often and financing global public campaigns against sexually transmitted diseases because digital health and molecular diagnostic advances are making testing more convenient and sensitive.The STD diagnostic market is growing considerably because of many significant factors. There is greater demand for early and accurate diagnosis because STDs are on the rise globally. Systematic testing is being promoted and public awareness is being enhanced through government programs, awareness campaigns, and education programs. Detection accuracy and speed are enhanced by advances in diagnostic technology, including molecular diagnostics and rapid point-of-care testing. Markets are supported by increased access to and infrastructure for healthcare, especially in developing countries. The expansion of the market and the growth of innovation are further supported by the increasing high-risk populations, wider acceptance of in-home testing kits, and the use of digital health solutions for tele-diagnostics.
Growth Drivers for the STD Diagnostic Market
Developments in diagnostic technologies
The market is growing due to developments in diagnostic technologies that have significantly enhanced the accessibility, efficiency, and accuracy of sexually transmitted disease (STD) detection. Rapid diagnostic tests, home testing kits, and nucleic acid amplification tests have all revolutionized STD diagnosis by providing convenient and efficient testing choices. These are beneficial in distant or remote areas with poor medical services accessibility. Investments in advanced diagnostic devices have grown with the increased requirement for precise and early detection methods. In June 2023, a spinout company from the University of Birmingham, Linear Diagnostics, was funded to develop a rapid STI test, the UK National Institute for Health and Care Research (NIHR) reported. In reaction to the urgent need for faster and simpler STI diagnosis, the company is working on a 20-minute test to diagnose gonorrhea and chlamydia. The market is growing due to these technical developments that are enhancing early detection efforts and enhanced patient outcomes.Government activities and raising awareness
Government actions and growing awareness are the major drivers of the STD diagnostics market growth. STIs are on the rise in many parts of the world, says recent research. WHO Member States had a target in 2022 to reduce the annual syphilis cases from 7.1 million to 0.71 million by 2030, a reduction of ten times. However, in 2022, 8 million new syphilis infections were reported among individuals aged between 15 and 49 years, up by over 1 million. The greatest increases were in the Americas and Africa. The necessity of reducing STI rates globally while ensuring that all people have access to prevention, care, and treatment has highly contributed to the demand for better and more accessible testing tools. The CDC's "Get Yourself Tested" program has also been essential in encouraging regular STD screening, among high-risk populations, and increasing awareness of the importance of early diagnosis. These markets are being fueled by a growing number of government-sponsored programs to inform the public and boost demand for STD screening services. The market will be fueled by increased focus on treatment and prevention and on regular testing.Growing incidence of sexually transmitted diseases
One of the primary driving forces for the necessity for improved diagnostic testing is the growing trend of sexually transmitted illnesses (STIs) globally. Rising infection numbers are brought about by several factors, including numerous sexual partners, unsafe sex, and not knowing the prevention techniques. An estimated one million people contract a STI daily according to the World Health Organization. One of the four primary STIs - trichomoniasis, syphilis, gonorrhea, or chlamydia - infects approximately 500 million individuals a year. The urgency of accurate and timely diagnosis treatments is brought out by the increasing prevalence of these diseases. Governments and healthcare organizations are also funding STI screening programs, which is also expected to grow demand for rapid and accurate diagnosis methods. Advances in self-testing kits, point-of-care testing, and molecular diagnostics have significantly contributed to market growth. Improving early detection, treatment results, and infection control measures has been high on the agenda of healthcare professionals.Challenges in the STD Diagnostic Market
Social Stigma and Privacy Concerns
The market for STD testing is severely hampered by social stigma and privacy issues. Underdiagnosis and untreated infections result from people avoiding testing out of fear of being judged, discriminated against, or having their privacy violated. This stigma is frequently a result of societal, religious, or cultural traditions that have a negative perception of STDs. People are deterred from getting tested at clinics or medical institutions by privacy concerns over sensitive health information. Consequently, there may be less demand for STD testing, especially in conservative or low-income areas. Increased knowledge, private testing choices, and the provision of discrete at-home testing kits are necessary to remove these obstacles and promote broader adoption and use.Regulatory Hurdles
The market for STD diagnostics is severely hampered by regulatory barriers, which postpone the approval and introduction of novel diagnostic procedures. The licensing procedure is complicated and time-consuming for producers because different nations have different and frequently strict regulatory standards. Adherence to safety assessments, clinical validation, and quality standards necessitates significant financial outlays, which can be especially taxing for smaller businesses. The market's growth is slowed by these delays, which restrict the supply of quick and creative diagnostic options. Furthermore, regionally disparate restrictions make it more difficult to enter international markets, raise prices, and prevent the broad use of cutting-edge STD detection tools.United States STD diagnostic Market
Growing awareness, increased STD prevalence, and improvements in testing technology are all contributing to the steady expansion of the US STD diagnostic industry. The FDA's Center for Biologics Evaluation and Research (CBER) authorized a labeling update for the OraQuick HIV Self-Test in January 2025, according to a statement released by OraSure Technologies, Inc. With this update, adolescents who were previously only eligible if they were 17 years of age or older can now be 14 years of age or older. As part of a larger public health initiative to reduce the spread of sexually transmitted diseases, this regulatory amendment seeks to increase HIV testing accessibility and early diagnosis in younger groups. In addition to conventional diagnostic techniques, the market continues to gain from new and easy-to-use self-testing kits.Germany STD diagnostic Market
The market for STD diagnostics in Germany is expanding gradually as a result of greater awareness, an increase in the frequency of STDs, and improvements in testing technology. The need for quick, precise, and user-friendly diagnostic solutions - such as point-of-care and home testing kits - is fueling the industry. To improve detection accuracy, top businesses concentrate on cutting-edge PCR-based and molecular diagnostics. Widespread STD screening and early diagnosis initiatives are supported by Germany's robust healthcare system and proactive public health regulations. All things considered, the market is anticipated to grow as consumers and healthcare professionals place a higher value on quick and easy STD testing alternatives.Australia STD diagnostic Market
The market for STD diagnostics in Australia is expanding rapidly due to rising STD incidence, more awareness, and the need for quick, precise testing methods. The market is growing as a result of government programs to encourage sexual health screening and advancements in diagnostic technology. BioLytical Laboratories Inc. launched the INSTI HIV-1/2 Syphilis Test in Australia in March 2025. With results in as little as 60 seconds, this cutting-edge dual test enables medical practitioners to detect syphilis and HIV with speed and connect patients to care. The Australian population is experiencing improved disease management and control as a result of the availability of such quick point-of-care diagnostics, which are improving early detection and treatment.Saudi Arabia STD diagnostic Market
Growing awareness, increased STD prevalence, and improvements in diagnostic technology are driving the Saudi Arabian STD diagnostic market's evolution. In order to support early detection and treatment, the government has put in place public health programs that encourage regular screening and sexual health education. The market gains from the use of home testing kits and point-of-care testing, which provide individuals seeking a diagnosis with privacy and convenience. Furthermore, partnerships between diagnostic firms and healthcare professionals are expanding the accessibility and availability of STD testing services nationwide. The Saudi Arabian market for STD diagnostics is therefore expected to continue expanding and growing.Recent Developments in STD Diagnostic Industry
- BioLytical Laboratories Inc. declared in March 2025 that its INSTI HIV-1/2 Syphilis Test would be available in Australia. In just 60 seconds, this dual HIV-syphilis test yields results, allowing medical providers to promptly diagnose HIV and syphilis and link patients to care.
- OraSure Technologies, Inc. reported in January 2025 that the FDA's Center for Biologics Evaluation and Research (CBER) had authorized a labeling update for the OraQuick HIV Self-Test. In contrast to its earlier approval for adults aged 17 and above, this update expands the age range of those eligible for the test to include adolescents aged 14 and above. The goal of this modification is to increase younger people's access to HIV testing.
- Mylab launched a range of quick tests for STD diagnosis in February 2023. These tests are easy to use, suitable for use in point-of-care facilities in resource-constrained locations, and can be stored at room temperature. They are also useful in blood banks for effectively detecting transfusion-transmissible illnesses (TTIs) in blood donors, which lowers the risk of transmission.
STD Diagnostic Market Segments:
Product
- Consumables
- Instruments& Services
- Software
Type
- Chlamydia Testing
- Gonorrhea Testing
- Herpes Simplex Virus Testing
- Human Papilloma Virus Testing
- Human Immunodeficiency Virus Testing
- Chancroid Testing
- Others
Location of Testing
- Laboratory Testing
- Point of Care Testing
Country
North America
- United States
- Canada
Europe
- France
- Germany
- Italy
- Spain
- United Kingdom
- Belgium
- Netherlands
- Turkey
Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Thailand
- Malaysia
- Indonesia
- New Zealand
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- South Africa
- United Arab Emirates
- Saudi Arabia
All companies have been covered from 5 viewpoints:
- Company Overview
- Key Persons
- Recent Development & Strategies
- SWOT Analysis
- Sales Analysis
Key Players Analysis
- Siemens Healthineers AG
- Abbott
- MedMira Inc
- QIAGEN
- Danaher Corporation (Cephied)
- F. Hoffmann-La Roche Ltd
- Diasorin SpA
- BioMeriuex
- Hologic Inc.
- Bio-Rad Laboratories Inc.
Table of Contents
Companies Mentioned
- Siemens Healthineers AG
- Abbott
- MedMira Inc
- QIAGEN
- Danaher Corporation (Cephied)
- F. Hoffmann-La Roche Ltd
- Diasorin SpA
- BioMeriuex
- Hologic Inc.
- Bio-Rad Laboratories Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | June 2025 |
| Forecast Period | 2024 - 2033 |
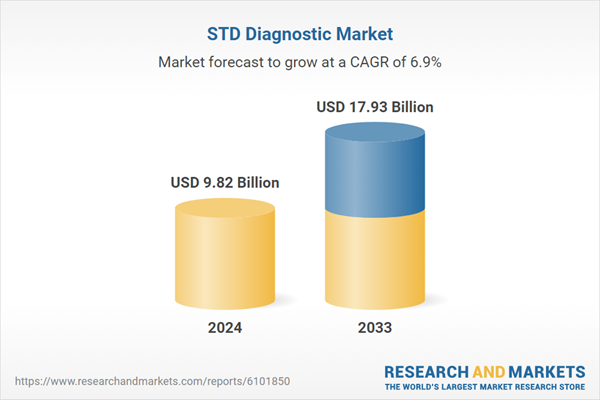
| Estimated Market Value ( USD | $ 9.82 Billion |
| Forecasted Market Value ( USD | $ 17.93 Billion |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |