Denmark Pharmaceutical Market Report by Therapeutic Class (Cancer, Infectious Diseases, Cardiovascular Diseases, Diabetes, Respiratory Diseases, Central Nervous System Disorders, Autoimmune Diseases, Others), Drug Type (Branded, Generic), Prescription Type (OTC Drugs, Prescription Drugs), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Others), and Company Analysis 2025-2033.
Denmark Pharmaceutical Market Overview
Investigation, research, production, and promotion of drugs and therapies utilized to cure, prevent, or control disease fall within the pharmaceutical sector. Through the provision of innovative medical treatments and increased life expectancy and quality of life, it plays an important role in world medicine. Scientific breakthroughs, government approvals, and the ongoing demand for effective treatments drive this industry. Both small biotech firms and international corporations are prominent players. Prescription drugs, vaccines, and over-the-counter drugs are all pharmaceuticals. The sector often collaborates with research and clinical institutions to accelerate development and is highly regulated to ensure safety and efficacy of drugs.High R&D expenditures, a collaborative academic and health environment, and supportive government regulations are the primary impetus for Denmark's pharmaceutical industry. Major contributions are made by leading global companies such as Novo Nordisk and Lundbeck through specialty medicine creation and global exports. Demand within the domestic market is also boosted by an aging population and the spread of chronic diseases such as diabetes. Innovation through personalized therapy and real-world evidence creation are enabled by advanced health data infrastructure in Denmark. Its global competitiveness is enhanced by an open regulatory system and access to EU markets. These combine to offer a robust, future-proof pharmaceutical sector with scope to expand.
Growth Drivers for the Denmark Pharmaceutical Market
Advanced Health Data Infrastructure
One of the key drivers for Denmark's pharmaceutical market's growth is its advanced heath data infrastructure. With biobanks, illness registries, and population-level electronic health records, the country boasts one of the most advanced and unified systems of healthcare data in the world. Since every Danish citizen is assigned a personal identification number, medical records can be exactly correlated between clinics, hospitals, and pharmacies. The approach opens up real-world data to academics and pharma companies to facilitate post-marketing surveillance, drug development, and clinical trials. Such access reduces the cost and time-to-market of research as well as speeds up pharmacovigilance and personalized treatment innovation. It also enhances the quality and efficiency of clinical trials by enabling long-term follow-up and targeted patient recruitment. In addition, Denmark is a safe destination for international collaboration due to the stringent data protection legislation that ensures patient confidentiality. This technological advantage enhances Denmark's competitiveness in the life sciences world and makes it a leading site for pharmaceutical R&D.Supportive Government Policies
Denmark's pharma industry is growing largely due to the favorable government policies of the country. Denmark has established a comprehensive Life Sciences Growth Plan incorporating 36 initiatives across six key areas such as clinical trials, R&D, and regulatory environments. This strategic plan aims to further consolidate Denmark's reputation as a leading life sciences nation by fostering innovation and streamlining procedures.The establishment of a "one-stop-shop" for drug companies that simplifies and quickens the regulatory approval process is an essential component of this program. By reducing administrative barriers, this program makes it easier for businesses to set up and expand production sites in Denmark. In addition, Denmark's climate agreement with the pharmaceutical industry illustrates its commitment to environmental responsibility. Consistent with international environmental aims, our partnership works to support green innovation and reduce CO2 emissions. Overall, taken as a package, all these beneficial government programs offer a good environment for pharmaceutical companies to expand, innovate, and become globally competitive for Denmark's pharmaceutical sector.
Strong R&D Investment
High investment in research and development (R&D) is one of the factors propelling the growth of the pharmaceutical market in Denmark. With a significant proportion being allocated to life sciences and pharmaceuticals, the country has some of the highest R&D expenditures per GDP of any nation globally. Both the public funding of the Danish government and substantial contributions from the private sector - particularly from major players such as Novo Nordisk and Lundbeck - funding this acquisition.Universities, hospitals, and enterprise are in close collaboration to facilitate Denmark's R&D environment that encourages innovation in biotechnology, personalized medicine, and drug development. Tax reductions, subsidies, and strategic initiatives such as the Life Sciences Growth Plan are some of the government measures that actively encourage R&D. Contemporary research infrastructure and highly skilled staff are also available, which adds to Denmark's competitive edge. This robust R&D climate accelerates the development of novel treatments, attracts foreign partnerships, and makes Denmark a world leader in clinical excellence and pharmaceutical innovation.
Challenges in the Denmark Pharmaceutical Market
Regulatory Complexity and EU Compliance
EU compliance and regulatory complexity provide major obstacles for Denmark's pharmaceutical industry. Businesses have to deal with two sets of regulations: strict European Union guidelines and Danish state laws. This results in a complicated and frequently drawn-out approval process for new medications and clinical studies, which can raise expenses and postpone market entry. Startups and small and medium-sized businesses (SMEs) frequently find it difficult to satisfy these standards due to the resource requirements and administrative load. Furthermore, ongoing adaptation is necessary due to the EU's periodic revisions and harmonization initiatives. For Danish pharmaceutical businesses, sustaining innovation speed while balancing compliance is still a major concern.Sustainability and Green Transition
For Denmark's pharmaceutical industry, sustainability and the green transition pose increasing obstacles. There is growing pressure on the business to lessen its environmental impact, which includes controlling water use in manufacturing processes, cutting carbon emissions, and limiting waste. Significant investments in cleaner technologies and process innovations are necessary to meet Denmark's aggressive climate targets and EU environmental requirements. Pharmaceutical businesses also need to strike a balance between sustainability measures and preserving cost-effectiveness and production efficiency. Changes in sourcing procedures and supply networks are also necessary for this shift. The Danish pharmaceutical industry must overcome the difficult task of successfully incorporating green practices while maintaining its competitiveness in order to keep up with worldwide sustainability trends.Recent Developments in Denmark Pharmaceutical Industry
- July 2022: The Danish business Bavarian Nordic increased its ability to produce 10 million doses of the monkeypox vaccine.
- June 2022: To encourage early identification of non-alcoholic steatohepatitis (NASH) and increase patient awareness, Novo Nordisk A/S, a leading worldwide healthcare organization, and Echosens, a high-tech company offering liver diagnostic instruments, teamed.
Denmark Pharmaceutical Market Segments:
Therapeutic Class
- Cancer
- Infectious Diseases
- Cardiovascular Diseases
- Diabetes
- Respiratory Diseases
- Central Nervous System Disorders
- Autoimmune Diseases
- Others
Drug Type
- Branded
- Generic
Prescription Type
- OTC Drugs
- Prescription Drugs
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Others
All companies have been covered from 5 viewpoints:
- Company Overview
- Key Persons
- Recent Development & Strategies
- SWOT Analysis
- Sales Analysis
Key Players Analysis
- Novo Nordisk A/S
- H. Lundbeck A/S
- Leo Pharma A/S
- Orifarm Group A/S
- ALK-Abell A/S
- Xellia ApS
- Takeda Pharma A/S
- Sandoz A/S
- Ferring Pharmaceuticals A/S
- FUJIFILM Diosynth Biotechnologies
Table of Contents
Companies Mentioned
- Novo Nordisk A/S
- H. Lundbeck A/S
- Leo Pharma A/S
- Orifarm Group A/S
- ALK-Abell A/S
- Xellia ApS
- Takeda Pharma A/S
- Sandoz A/S
- Ferring Pharmaceuticals A/S
- FUJIFILM Diosynth Biotechnologies
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | June 2025 |
| Forecast Period | 2024 - 2033 |
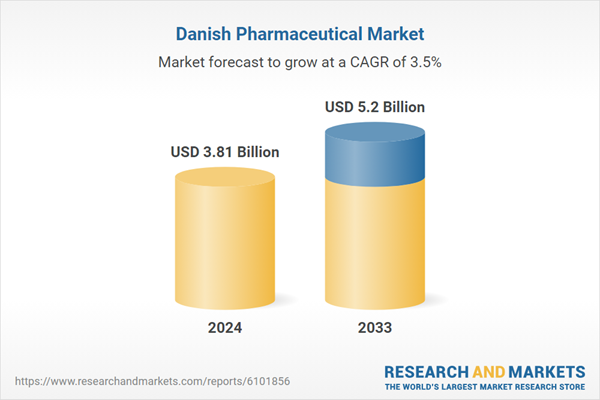
| Estimated Market Value ( USD | $ 3.81 Billion |
| Forecasted Market Value ( USD | $ 5.2 Billion |
| Compound Annual Growth Rate | 3.5% |
| Regions Covered | Denmark |
| No. of Companies Mentioned | 10 |