Japan Inflammatory Bowel Disease Treatment Market Report by Drug Class (TNF Inhibitors, Anti-Integrin, IL inhibitors, JAK inhibitors, Corticosteroids, Aminosalicylates, Others), Disease Indication (Ulcerative Colitis, Crohn's Disease), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy) and Company Analysis, 2025-2033.
Japan Inflammatory Bowel Disease Treatment Industry Overview
The increasing frequency of diseases like Crohn's disease and ulcerative colitis has led to significant increase in the Japanese market for inflammatory bowel disease (IBD) treatments. The patient pool is rising as a result of increased awareness, better diagnostic methods, and an aging population. The adoption of novel medicines is encouraged by Japan's healthcare system, which is renowned for its sophisticated infrastructure and strong emphasis on providing high-quality care. A wide variety of therapy alternatives are available on the market, ranging from conventional corticosteroids and immunosuppressants to innovative biologic medications such as Janus kinase (JAK) inhibitors, anti-integrins, and anti-TNF medicines. With their ability to provide more focused and efficient disease care, especially for patients with moderate to severe IBD, these biologics have completely changed the therapeutic landscape. Patient access to these cutting-edge therapies has also been greatly aided by the government's emphasis on improving healthcare cost and accessibility through insurance coverage and subsidy schemes.Strong research and development (R&D) efforts in Japan, spearheaded by both local pharmaceutical firms and international enterprises doing business there, are another advantage for the sector. In an effort to increase effectiveness and reduce side effects, ongoing innovation is driving the development of novel treatments, such as small molecule medications and next-generation biologics. With customized treatment plans that enhance patient outcomes and save long-term healthcare expenditures, personalized medicine is becoming more and more popular. Notwithstanding this expansion, the industry still confronts obstacles including the high price of biologic treatments, low patient knowledge that causes diagnosis to be delayed, and unequal access in urban and rural regions. Market dynamics are further impacted by safety issues and regulatory barriers associated with long-term biologic usage.
However, these obstacles are being addressed by strategic partnerships among government agencies, academic institutions, and industrial players. All things considered, Japan's IBD treatment market is poised for sustained expansion as the need for efficient and patient-centered treatments is fueled by advancements, government assistance, and growing illness awareness.
Key Factors Driving the Japan Inflammatory Bowel Disease Treatment Market Growth
Advancements in Treatment Options
The market for IBD treatments in Japan has grown significantly as a result of improvements in therapeutic choices, especially with the advent of biologic medications. Anti-TNF (tumor necrosis factor) drugs, anti-integrin medications, and JAK (Janus kinase) inhibitors are examples of biologic therapeutics that have transformed the treatment of inflammatory bowel disorders, including Crohn's disease and ulcerative colitis. Compared to conventional treatments, these tailored therapeutics help lower inflammation more successfully by altering particular immune system pathways. For patients with moderate to severe IBD, they provide higher quality of life, longer remission times, and increased effectiveness. These biologics' increasing accessibility and acceptability, together with Japan's encouraging reimbursement practices, are what keep their use rising and propelling the industry.Personalized Medicine Approaches
The market for IBD treatments in Japan is changing dramatically due to personalized medicine. Personalized therapy options take into account each patient's unique features, including genetics, the severity of the disease, response to prior therapies, and comorbidities, rather than employing a one-size-fits-all strategy. By choosing the best treatment with the fewest side effects, this precision-based method enhances clinical results and patient happiness. More precise evaluations and treatment planning are made possible by developments in diagnostic technologies, such as biomarkers and genetic testing. When treating chronic and complicated conditions like IBD, where treatment outcomes can differ greatly, this customized approach is very helpful. The influence of customized medicine on enhancing treatment and raising market demand for targeted medicines is becoming more apparent as healthcare systems and providers embrace it more and more.Research and Development Initiatives
One of the main factors propelling the Japanese market for IBD treatments is ongoing research and development (R&D). In order to provide novel treatments that meet unmet clinical requirements and enhance patient outcomes, pharmaceutical corporations and academic institutions are aggressively investing in research and development. These initiatives include investigating novel small chemicals, biologic medicines, and treatments based on the microbiome. The body of data supporting the effectiveness and safety of treatments is also growing as a result of clinical trials and real-world research. Japan's dedication to medical innovation and its lenient regulatory framework promotes the quick development and clearance of new medications. Partnerships between domestic and international businesses also aid in quickening the pace of innovation. Long-term improvements in patient care and market competitiveness are made possible by this strong emphasis on research and development, which guarantees a consistent flow of innovative treatment choices.Challenges in the Japan Inflammatory Bowel Disease Treatment Market
High Cost of Biologic Therapies
Because biologic medicines provide tailored and effective choices, particularly for moderate to severe cases, they have revolutionized the therapeutic landscape for inflammatory bowel disease (IBD). However, the hefty cost of these cutting-edge therapies makes them extremely difficult for many Japanese patients to afford. In addition to having an effect on individual patients, the high expenses put a great deal of strain on the nation's insurance and healthcare budgets. Even with government initiatives to offer insurance and subsidies, out-of-pocket costs might still be high. Some patients may turn to outdated or less effective treatments as a result of this price barrier, which may restrict their access to the best medicines. Because of this, the high price of biologics continues to be a major barrier to their broad use and fair treatment for all patient populations.Limited Awareness and Late Diagnosis
The general public's and even some healthcare professionals' lack of knowledge about the early signs and severity of inflammatory bowel disease (IBD) is a significant management concern in Japan. Non-specific symptoms including weariness, diarrhea, and stomach discomfort are common in the disease and might be confused with less serious illnesses. This ignorance causes the condition to worsen and inflict more serious intestinal damage by delaying diagnosis and therapy commencement. A delayed diagnosis raises the risk of complications, hospital stays, and procedures in addition to decreasing the efficacy of currently available treatments. Improving patient outcomes and lowering the overall burden of IBD need strengthening public education campaigns and educating medical professionals on how to spot early symptoms.Market Segmentations
Drug Class
- TNF Inhibitors
- Anti-Integrin
- IL inhibitors
- JAK inhibitors
- Corticosteroids
- Aminosalicylates
- Others
Disease Indication
- Ulcerative Colitis
- Crohn's Disease
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
All the Key players have been covered
- Overview
- Key Persons
- Recent Developments
- Revenue Analysis
Company Analysis:
- Abbott Laboratories
- AbbVie Inc.
- Allergan Therapeutics LLC
- Bausch Health Companies Inc. (Salix Pharmaceuticals)
- Bristol-Myers Squibb Company
- Johnson & Johnson
- Novartis AG
- Pfizer Inc.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- AbbVie Inc.
- Allergan Therapeutics LLC
- Bausch Health Companies Inc. (Salix Pharmaceuticals)
- Bristol-Myers Squibb Company
- Johnson & Johnson
- Novartis AG
- Pfizer Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | June 2025 |
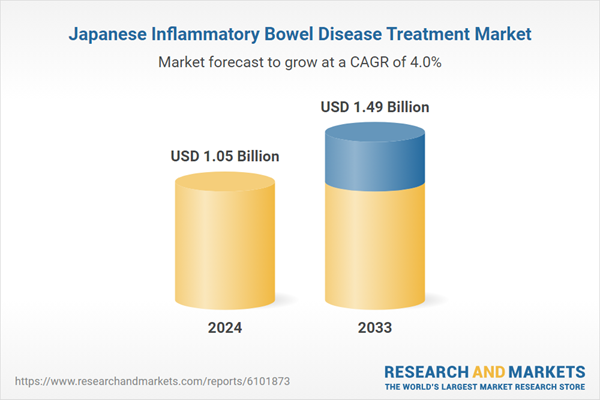
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 1.05 Billion |
| Forecasted Market Value ( USD | $ 1.49 Billion |
| Compound Annual Growth Rate | 3.9% |
| Regions Covered | Japan |
| No. of Companies Mentioned | 8 |