United States Personalized Medicine Market Report by Product (Personalized Medicine Therapeutics, Personalized Medicine Diagnostics, Personalized Medical Care, Personalized Nutrition and Wellness), Application (Oncology, Infectious disease, Neurology or Psychiatry, Cardiovascular, Others), End-User (Hospitals, Diagnostic Centers, Research & Academic Institutes, Others) and Company Analysis, 2025-2033.
United States Personalized Medicine Market Overview
Innovations in digital health, biotechnology, and genomics are propelling the revolutionary expansion of the customized medicine sector in the United States. The goal of personalized medicine is to adjust medical care to each patient's unique traits, such as their environment, lifestyle, and genetic composition. With this strategy, healthcare is moving away from the conventional "one-size-fits-all" paradigm and toward more accurate, predictive, and preventative treatment. Healthcare providers and pharmaceutical firms are investing more in research and development to provide treatments with better efficacy and fewer side effects as a result of the increased emphasis on customized therapy.The incorporation of advanced technologies like artificial intelligence, machine learning, and next-generation sequencing into clinical and research settings is a major factor driving this industry. Clinicians can use these technologies to find biomarkers, genetic mutations, and other molecular signs that help with diagnostic and treatment choices. The capacity to match patients with tailored therapies is also being improved by the growing availability of companion diagnostics, especially in intricate domains like neurology and cancer. Because of this, customized medicine is changing clinical procedures, encouraging early intervention, and facilitating better illness management in a variety of therapeutic domains.
The industry is also being driven ahead by government assistance, changing regulations, and cooperation between the public and private sectors. The infrastructure required for the widespread adoption of customized healthcare is being built in part by initiatives to gather and analyze a variety of patient data. Additionally, healthcare practitioners are being prompted to embrace more patient-centric methods due to rising patient awareness and desire for customized treatment alternatives. The United States is now positioned as a worldwide leader in customized medicine, and these advancements are fostering an atmosphere that is conducive to innovation. Cost, data privacy, and accessibility issues still exist as the sector develops, but sustained cooperation and advancements in technology could help to overcome these obstacles and spur further expansion.
Key Factors Driving the United States Personalized Medicine Market Growth
Advancements in Genomic and Molecular Technologies
Advances in molecular diagnostics, bioinformatics, and genome sequencing are greatly speeding up the use of customized medicine in the United States. These technologies make it possible to identify biomarkers and genetic variants that aid in customizing treatment regimens for each patient. Patient genomes may now be analyzed more quickly and affordably thanks to next-generation sequencing and other high-throughput technologies. This reduces trial-and-error in therapy selection, which improves treatment results in addition to improving diagnostic accuracy. These technologies are being incorporated into standard clinical practice as they become more affordable and available, especially in areas like pharmacogenomics, uncommon illnesses, and cancer. This technical advancement is still a significant driver of market expansion for biopharmaceutical businesses, hospitals, and research facilities.Growing Demand for Targeted Therapies
More and more patients and medical professionals are looking for treatments that are tailored to each patient's unique health profile, especially for chronic or complicated illnesses like diabetes, autoimmune diseases, and cancer. A move toward more accurate, focused therapy has resulted from the unintended side effects and variable results of traditional treatments. In order to improve efficacy and safety, personalized medicine uses genetic and molecular data to anticipate a patient's response to certain therapies. Pharmaceutical firms are investing more in biologics and companion diagnostics as a result of the desire for customized treatment, which is also having an impact on drug development pipelines. The need for customized medicine is rising in all healthcare settings as more people become aware of the advantages of tailored care.Supportive Regulatory and Policy Environment
The United States' regulatory environment has shifted in favor of customized medicine, fostering innovation and expediting the approval of focused treatments. Frameworks and criteria for assessing companion diagnostics and precision medicines have been created by organizations like the U.S. Food and Drug Administration (FDA). A solid basis for market expansion has been established by initiatives including national genome projects, public-private collaborations, and federal support for precision medicine research. The goal of these initiatives is to hasten the shift from conventional to individualized care models. Furthermore, the financial feasibility of customized medicine solutions is being further enhanced by continuous improvements in healthcare payment systems that are gradually opening up individualized therapies to a wider public.Challenges in the United States Personalized Medicine Market
High Costs of Development and Treatment
Costs associated with personalized medicine are high, especially when it comes to research, genetic testing, and medication development. A substantial investment in clinical trials, genetic research, and regulatory approval procedures is necessary to develop customized treatments. Furthermore, many patients cannot afford the exorbitant cost of many customized treatments, particularly those involving biologics or cell and gene therapies. Additionally, the expense of sophisticated sequencing technology and companion diagnostics may prevent widespread usage in routine clinical settings. Both individuals and healthcare providers have financial difficulties as a result of these high costs, which frequently lead to inadequate insurance coverage. The high cost of customized medicine continues to be a significant obstacle to its wider adoption throughout the American healthcare system until more scalable and reasonably priced models are created.Limited Standardization and Integration in Clinical Practice
Personalized medicine has not yet been completely incorporated into standard clinical care, despite its potential. The lack of uniformity in the interpretation and use of genomic data is a significant obstacle. Complex genetic information can be difficult for healthcare professionals to comprehend and use, especially in basic care settings. Furthermore, a large number of electronic health records (EHRs) lack the necessary tools for effectively managing or integrating genetic data. The lack of qualified experts, such as precision medicine specialists and genetic counselors, is another issue impeding broad adoption. The actual implementation of customized medicine in healthcare facilities is still uneven in the absence of uniform procedures, training, and smooth technology integration.Market Segmentations
Product
- Personalized Medicine Therapeutics
- Personalized Medicine Diagnostics
- Personalized Medical Care
- Personalized Nutrition and Wellness
Application
- Oncology
- Infectious disease
- Neurology or Psychiatry
- Cardiovascular
- Others
End User
- Hospitals
- Diagnostic Centers
- Research & Academic Institutes
- Others
All the Key players have been covered
- Overview
- Key Persons
- Recent Developments
- Revenue Analysis
Company Analysis:
- Abbott Laboratories
- GE Healthcare, Inc.
- Aadi Bioscience, Inc.
- Illumina, Inc.
- QIAGEN
- Eli Lilly and Company
- Takeda Pharmaceutical Company Ltd
- AbbVie Inc.
- F. Hoffmann-La Roche Ltd.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- GE Healthcare, Inc.
- Aadi Bioscience, Inc.
- Illumina, Inc.
- QIAGEN
- Eli Lilly and Company
- Takeda Pharmaceutical Company Ltd
- AbbVie Inc.
- F. Hoffmann-La Roche Ltd.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | June 2025 |
| Forecast Period | 2024 - 2033 |
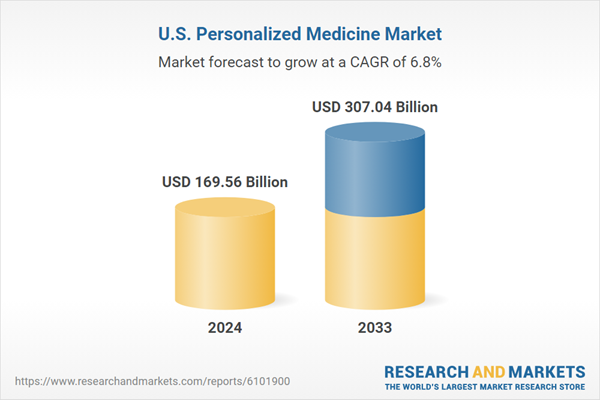
| Estimated Market Value ( USD | $ 169.56 Billion |
| Forecasted Market Value ( USD | $ 307.04 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | United States |
| No. of Companies Mentioned | 9 |