United States Epilepsy Drugs Market Report by Drugs Category (First Generation Drugs, Second Generation Drugs, Third Generation Drugs), Seizure Types (Focal Seizures, Generalized Seizures, Non-Epileptic Seizures), Distribution Channels (Hospital Pharmacies, Drug Stores and Retail Pharmacies, Online Providers) and Company Analysis, 2025-2033.
United States Epilepsy Drugs Industry Overview
The market for epilepsy medications in the United States is a vibrant area of the larger neurology therapeutics business, with a wide range of antiepileptic medications (AEDs) that target different kinds of seizures. North America accounted for the greatest portion of the worldwide market for epilepsy medications in 2024, with the United States leading the way in terms of market size and revenue production. Due to their enhanced safety profiles and effectiveness, second-generation AEDs are growing at the quickest rate in the market, which is divided into first, second, and third-generation AEDs. The high demand for efficient treatment solutions is shown by the estimated 1.2% prevalence of epilepsy in the United States.The prevalence of epilepsy has increased in the U.S. population. The seizures Foundation estimates that more than 3.4 million Americans suffer from seizures. Furthermore, 1 in 26 Americans are predicted to receive a diagnosis of epilepsy at some point in their lives, with over 150,000 receiving a diagnosis each year.
New developments in medication development have accelerated the market's growth even further. The FDA's 2022 approval of ganaxolone (Ztalmy) for the treatment of seizures linked to CDKL5 deficient condition was a noteworthy milestone that provided a new therapeutic option for a patient population that had previously been underserved. Furthermore, the growing investment in specialist epilepsy medicines is demonstrated by Lundbeck's $2.6 billion acquisition of Longboard Pharmaceuticals in 2024, which was intended to develop bexicaserin for the treatment of developmental and epileptic encephalopathies. These advancements are in line with the demand for treatments that target complicated and uncommon epilepsy disorders as well as the increased emphasis on customized therapy.
Key Factors Driving the United States Epilepsy Drugs Market Growth
Increasing Prevalence of Epilepsy
One of the main factors propelling market growth is the increasing prevalence of epilepsy in the United States. It affects around 2.9 million individuals who are 18 years of age or older, with a significant incidence in youngsters. The need for efficient anti-epileptic medications (AEDs) is increased by this expanding patient base. Certain demographics, such as those from minority racial and ethnic groups and those with lower educational attainment, are more likely to have the syndrome. As more people look for medical intervention and treatment alternatives, the market is growing as a result of growing awareness and diagnosis of epilepsy. In order to satisfy the needs of a varied patient population, this trend emphasizes the necessity of ongoing innovation and accessibility in the creation and distribution of AEDs.Advancements in Drug Development
Second and third-generation anti-epileptic medications (AEDs), which have better effectiveness and safety profiles, have been introduced as a result of ongoing research and development efforts. These more recent drugs aim to overcome the drawbacks of previous therapies by improving seizure control while reducing adverse effects. For example, the FDA approved UCB's FINTEPLA (fenfluramine) oral solution in 2022 to treat Lennox-Gastaut syndrome-related seizures in individuals two years of age and older. These developments not only improve patient outcomes but also provide medical professionals more treatment alternatives. The dedication to meeting the unmet requirements of the epilepsy community is seen in the ongoing pipeline of new AEDs, which propels market expansion.Favorable Regulatory Environment
Faster access to new medicines is made possible by the U.S. Food and Drug Administration's (FDA) accelerated approval of innovative anti-epileptic medications (AEDs). Pharmaceutical firms are encouraged to invest in the discovery of novel medicines by this regulatory assistance. Furthermore, programs like the Epilepsy Foundation's #RemoveTheFilter and National Epilepsy Awareness Month have raised public awareness and decreased stigma, which has encouraged more people to seek treatment. Because of these initiatives, the patient population is more informed, which increases the likelihood of diagnoses and treatment compliance. The market for epilepsy medications is expected to develop in a favorable environment because to a mix of supportive regulatory frameworks and increased public awareness.Challenges in the United States Epilepsy Drugs Market
High Treatment Costs and Affordability Issues
Access to therapy is severely hampered by the rising costs of antiepileptic medications (AEDs) in the US. For example, generic drugs are frequently less expensive than name-brand ones, such as Fycompa and Aptiom, which may cost thousands of dollars a month. Generics are not always readily available, and some more recent AEDs do not have generic counterparts, which keeps their prices high. Furthermore, insurance coverage might vary; Medicare seniors are frequently not eligible for co-pay assistance programs, and other plans have hefty deductibles or co-pays. These monetary difficulties may cause patients to stop taking their medications, which raises the risk of seizures and related problems. In order to provide fair access to efficient epilepsy therapies, it is imperative that these economic concerns be addressed.Regulatory Hurdles and Market Entry Delays
New antiepileptic medications (AEDs) must pass rigorous regulatory criteria enforced by the U.S. Food and Drug Administration (FDA), which include thorough documentation and lengthy clinical studies. For pharmaceutical firms, this stringent procedure may raise development costs and postpone the release of new medications. Furthermore, generic versions of important AEDs are introduced when their patents expire; although they are less expensive, they might not always satisfy the unique demands of every patient. People who need specialized care may have fewer alternatives due to these market factors. Additionally, market access and the profitability of AED development initiatives may be hampered by the drawn-out and expensive approval procedures linked to regulatory obstacles.Market Segmentations
Drugs Category
- First Generation Drugs
- Second Generation Drugs
- Third Generation Drugs
Seizure Types
- Focal Seizures
- Generalized Seizures
- Non-Epileptic Seizures
Distribution Channel
- Hospital Pharmacies
- Drug Stores and Retail Pharmacies
- Online Providers
All the Key players have been covered
- Overview
- Key Persons
- Recent Developments
- Revenue Analysis
Company Analysis:
- Eisai Co., Ltd.
- UCB Inc.
- H. Lundbeck A/S
- GW Pharmaceuticals Plc.
- Abbott Laboratories
- Alkem Laboratories Limited
- Bausch Health Companies Inc.
- GSK plc.
Table of Contents
Companies Mentioned
- Eisai Co., Ltd.
- UCB Inc.
- H. Lundbeck A/S
- GW Pharmaceuticals Plc.
- Abbott Laboratories
- Alkem Laboratories Limited
- Bausch Health Companies Inc.
- GSK plc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | June 2025 |
| Forecast Period | 2024 - 2033 |
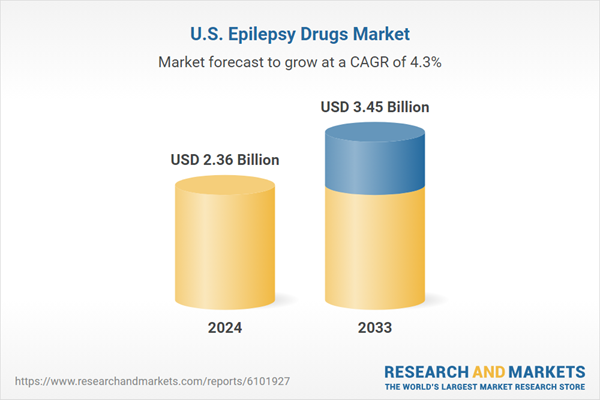
| Estimated Market Value ( USD | $ 2.36 Billion |
| Forecasted Market Value ( USD | $ 3.45 Billion |
| Compound Annual Growth Rate | 4.3% |
| Regions Covered | United States |
| No. of Companies Mentioned | 8 |