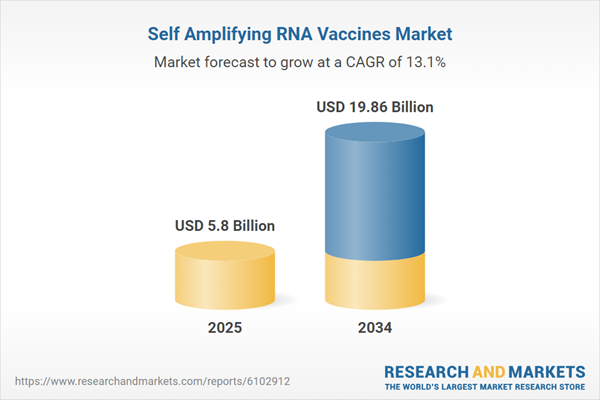
Self Amplifying RNA Vaccines Market Overview
Self-amplifying RNA (saRNA) vaccines use a self-replicating RNA platform to generate immune responses in the body. RNA vaccines work by triggering the immune system to defend against specific pathogens, using messenger RNA instead of inactivated viruses. Self-amplifying RNA vaccines are a specialized type of RNA vaccine that allows the RNA to replicate within the host cells. This self-amplification process leads to a strong immune response and higher levels of protein production. saRNA vaccine produces enhanced immunogenicity, and lower doses are required due to its self-amplifying nature and rapid production. This continues to drive the development of saRNA vaccine technology.Self Amplifying RNA Vaccines Market Growth Drivers
FDA Clearances Being Instrumental in Rising Market Demand
The market growth is driven by the evolving regulatory landscape that is allowing high-efficacy vaccines and medications in the market to address unmet needs for a wider section of the population. For instance, in April 2024, Ractigen Therapeutics, a pioneer in small activating RNA (saRNA) therapeutic development, received approval from the U.S. Food and Drug Administration for its Investigational New Drug application concerning RAG-01. This innovative saRNA treatment is expected to treat non-muscle invasive bladder cancer. This FDA approval is poised to commence clinical trials in several other regions along with the United States, bolstering market growth as regulatory approvals and clinical trials play a vital role in adding credibility to new drugs.Additionally, the world’s first saRNA vaccine ARCT-154 was approved by the Japanese regulatory authorities in Japan in November 2023. This approval has given access to a new weapon to healthcare providers, through which the prevention of infectious diseases, and management of chronic diseases such as cancer will be made easier than ever. This approval marked a significant milestone as the first saRNA vaccine to be registered in the world.
Self Amplifying RNA Vaccines Market Trends
_x000D_The market is witnessing several trends and developments to improve the global current scenario. Some of the notable trends are as follows:
Self Amplifying RNA Vaccines Market Segmentation
Market Breakup by Route of Administration:
- Intradermal
- Intranasal
- Intramuscular
- Others
Market Breakup by Application:
- Influenza
- Rabies
- HIV-1
- COVID-19
- Respiratory Syncytial Virus (RSV)
- Ebola
- Others
Market Breakup by Region:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Self Amplifying RNA Vaccines Market Share
Market Segmentation Based on Application to Witness Significant Growth
Based on application, the market report includes influenza, rabies, HIV-1, COVID-19, respiratory syncytial virus (RSV), and Ebola, among others. Historically, the COVID-19 segment has had a significant share of the market, driven by the advent of an unforeseen pandemic which triggered an urgent demand for effective vaccines, and led to the quick development of RNA-based vaccines globally. The market segment is backed by heavy investments by the government and several key players in the market.Self Amplifying RNA Vaccines Market Analysis by Region
Based on region, the market is divided into North America, Europe, Asia Pacific, Latin America and the Middle East and Africa. Out of these, North America is the leading market due to the presence of global players in the region. The market dominance can be attributed to factors technical innovations as well as product launches.Europe, with the presence of prominent academic institutions, holds a significant market share. In addition, the Asia Pacific region is expected to witness substantial growth in the forecast period. The rapid growth can be attributed to rising investments in improving the healthcare infrastructure to boost research and development initiatives related to the market. Countries like India, Japan and China are expected to emerge as key regional players in the Asia Pacific market._x000D_
Leading players in the Self Amplifying RNA Vaccines Market
The key features of the market report comprise patent analysis, clinical trials analysis, grants analysis, funding and investment analysis along with strategic initiatives by the leading players. The major companies in the market are as follows:Pfizer Inc.
Pfizer is a global leader in developing and producing vaccines for immunology, oncology, cardiology, endocrinology, and neurology. The company played a pivotal role in the COVID-19 pandemic. The company partnered with BioNTech to study and develop COVID-19 mRNA vaccine candidates during the pandemic and delivered millions of vaccines.Gennova Biopharmaceuticals Limited
The company is headquartered in Pune, India. Gennova is a biotechnology company focused on the research, development, production, and commercialization of bio-therapeutics and vaccines to address life-threatening diseases across various indications.Arcturus Therapeutics, Inc.
It is an American RNA medicines biotechnology company focused on finding, developing, and delivering treatments for rare diseases. Arcturus has developed proprietary lipid nanoparticle RNA therapeutics for nucleic acid medicines including small interfering RNA (siRNA), messenger RNA (mRNA), gene editing RNA, DNA, antisense oligonucleotides, and microRNA.VLP Therapeutics Japan, LLC
VLP Therapeutics Japan, LLC specialises in developing innovative vaccines and therapeutics using their proprietary Virus-Like Particle (VLP) technology. Their portfolio includes cutting-edge vaccines for infectious diseases such as dengue fever, malaria, and COVID-19, aimed at providing effective and long-lasting immunity with enhanced safety profiles.CSL Limited
It is an Australian biotechnology company focused on the research, development, and commercialisation of products that treat and prevent serious human medical conditions. The company portfolio includes blood plasma derivatives, vaccines, antivenom, and cell culture reagents used in various medical and genetic research and manufacturing applications.Other key players in the market include TriLink BioTechnologies, ImmunityBio, Inc., Gritstone bio, Inc., and eTheRNA Immunotherapies NV.
Key Questions Answered in the Self Amplifying RNA Vaccines Market
- What was the global self amplifying RNA vaccines market value in 2024?
- What is the global self amplifying RNA vaccines market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on application?
- How is the market segmented based on the route of administration?
- What are the major factors aiding the self amplifying RNA vaccines market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major self amplifying RNA vaccines market trends?
- Which route of administration is expected to dominate the market?
- Which application will dominate the market share?
- Who are the key players involved in the self amplifying RNA vaccines market?
- What is the patent landscape of the market?
- How many clinical trials are being conducted for self amplifying RNA vaccines?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Gennova Biopharmaceuticals Limited
- Arcturus Therapeutics, Inc.
- VLP Therapeutics Japan, LLC
- CSL Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 5.8 Billion |
| Forecasted Market Value ( USD | $ 19.86 Billion |
| Compound Annual Growth Rate | 13.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |