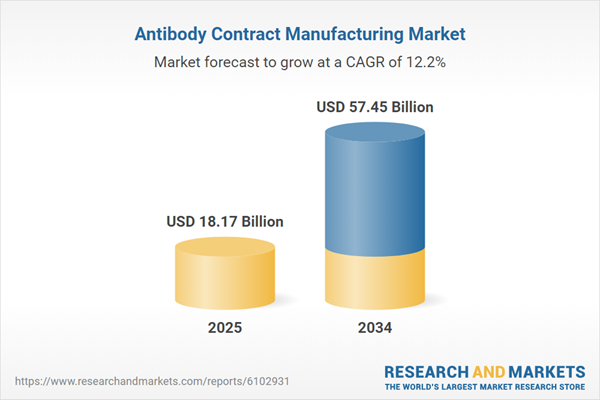
Antibody Contract Manufacturing Market Overview
Antibody contract manufacturing involves the production of biologic therapies, including monoclonal antibodies (mAb), polyclonal antibodies, and others. These therapeutics target specific antigens to treat cancer, autoimmune disorders, and infectious diseases. With advancements in mAbs, bispecific antibodies, and antibody-drug conjugates (ADCs), the market is poised for substantial growth in the forecast period.Antibody Contract Manufacturing Market Growth Drivers
Rising Prevalence of Chronic Diseases is Likely to Drives Market Growth
According to the Centers for Disease Control and Prevention (CDC) 2024 report, approximately 129 million people in the United States (including children and adults) are affected by one or more major chronic illnesses including heart disease, cancer, diabetes, obesity, and hypertension illnesses. The rising prevalence of chronic diseases is driving market demand for antibody contract manufacturing as the need for effective therapeutic alternatives against chronic diseases is on the rise.Antibody Contract Manufacturing Market Trends
Several trends and developments are being observed in the market to enhance the current situation. Some of the noteworthy trends are as follows:
Surge in FDA Approvals to Boost Market Growth
In June 2023, UCB's RYSTIGGO® (rozanolixizumab-noli) received FDA approval under the Priority Review designation. This humanized IgG4 monoclonal antibody eliminates the symptoms of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AchR) or anti-muscle-specific tyrosine kinase (MuSK) antibody positive. The market is witnessing a surge in drug approvals that involve monoclonal antibodies. This can be attributed to the increasing adoption of these therapies for treating and managing rare and chronic diseases. This, in turn, is boosting the demand for antibody contract manufacturing.Rising Preference for Monoclonal Antibodies (mAbs)
Monoclonal antibodies are gaining preference due to their targeted approach and effectiveness in treating various diseases, particularly in oncology and autoimmune disorders. Their ability to target specific substances helps reduce side effects, contributing to their appeal in targeted treatments.
Growing Demand for Bispecific Antibodies to Boost the Antibody Contract Manufacturing Market Demand
Bispecific antibodies, designed to bind to two distinct antigens simultaneously, are gaining traction in the market due to their ability to provide dual-action therapeutic benefits. They are especially effective in treating complex diseases, such as cancer, and are opening new possibilities in antibody design and advanced treatments, thereby accelerating the demand for antibody contract manufacturing.Adoption of Antibody-Drug Conjugates (ADCs) for Cancer Treatment to Boost Antibody Contract Manufacturing Market Value
Antibody-drug conjugates (ADCs) are transforming cancer treatment by directly delivering potent drugs to tumor cells, minimizing harm to normal tissues, and improving treatment efficacy. This precision is revolutionizing cancer treatment, positioning ADCs as a key focus area in the market.Antibody Contract Manufacturing Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:Market Breakup by Product
- Monoclonal Antibodies
- Polyclonal Antibodies
- Others
Market Breakup by Source
- Mammalian
- Microbial
Market Breakup by Therapeutic Area
- Cardiology
- Oncology
- Infectious Diseases
- Immune Disorders
- Neurology
- Others
Market Breakup by End User
- Biopharmaceutical and Pharmaceutical Companies
- Research Laboratories
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Antibody Contract Manufacturing Market Share
Market Segmentation Based on the Product is Anticipated to Witness Substantial Growth
Based on product, the market is divided into monoclonal antibodies, polyclonal antibodies, and others. Among these, the monoclonal antibody segment leads the market share due to its extensive applications in oncology, immunology, and therapies for autoimmune conditions. The market share for targeted therapies is expected to grow further as its adoption witnesses an increase and biotechnology advances.Antibody Contract Manufacturing Market Analysis by Region
Regionally, the market report offers an insight into North America, Europe, Asia Pacific, Latin America, the Middle East, and Africa. Among these, North America is expected to dominate the market because of the presence of major pharmaceutical companies and substantial healthcare expenditures. Intensive research efforts and government support for cancer research are fueling the expansion of the domestic market.Leading Players in the Antibody Contract Manufacturing Market
The key features of the market report include patent analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies are:Lonza
Founded in 1897, this company is headquartered in Basel, Switzerland, it is one of the largest companies providing a wide range of antibody contract manufacturing services, with a focus on monoclonal antibody production. Some of their products include Darzalex® (daratumumab), and Avastin (bevacizumab), among others. The company offers mammalian cell culture technologies, process development, and commercial manufacturing on an industrial scale.Thermo Fisher Scientific Inc.
Founded in 2006, Thermo Fisher Scientific is based in Waltham, Massachusetts, USA. The company offers a broad range of services in antibody contract manufacturing and produces antibodies like Repatha® (evolocumab) and Imfinzi® (durvalumab). Thermo Fisher supports the biopharma industry with its integrated services, including antibody production, purification, and final formulation.WuXi Biologics (Cayman) Inc.
Founded in 2010 and headquartered in Wuxi, China, they have produced antibodies like Imfinzi® (durvalumab) and Bavencio® (avelumab). WuXi offers services ranging from the development of the cell line to commercial-scale antibody production for global markets throughout Europe, North America, and the rest of the world.FUJIFILM Holdings Corporation
FUJIFILM Holdings is a leading company in the manufacture of antibody contract services, established in 1934 and headquartered in Tokyo, Japan. Their products include Opdivo (nivolumab) and Yervoy (ipilimumab). Other services involved in the market include biologics and commercial-scale production of monoclonal antibodies.Other companies include Samsung Biologics Co., Ltd., Charles River Laboratories, AGC Biologics, Cytovance Biologics, Inc., Emergent BioSolutions, Labcorp Drug Development, Catalent, Inc., and Boehringer Ingelheim Biopharmaceuticals GmbH.
Key Questions Answered in the Global Antibody Contract Manufacturing Market
- What was the global antibody contract manufacturing market value in 2024?
- What is the global antibody contract manufacturing market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is the market segmentation based on the product?
- What is the market breakup based on the source?
- What is the market segmentation based on therapeutic area?
- What is the market segmentation based on the end user?
- What are the major factors aiding the antibody contract manufacturing market demand?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major antibody contract manufacturing market trends?
- How does the rise in the geriatric population impact the market size?
- Who are the key players involved in the antibody contract manufacturing market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
- Which indication dominates the antibody contract manufacturing market?
- How is the Asia Pacific region contributing to the growth of the antibody contract manufacturing market?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Lonza
- Thermo Fisher Scientific Inc.
- WuXi Biologics (Cayman) Inc.
- FUJIFILM Holdings Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 18.17 Billion |
| Forecasted Market Value ( USD | $ 57.45 Billion |
| Compound Annual Growth Rate | 12.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |