As of May 2025, there are 937 Diagnostic centers in Nigeria, representing a 3.65% increase from 2023. Of these locations, 899 Diagnostic centers, which is 95.94% of all Diagnostic centers in Nigeria, are single-owner operations, while the remaining 38, 4.06%, are part of larger brands. The top three states with the most Diagnostic centers are Lagos, with 256 Diagnostic centers; Federal Capital Territory, with 104 Diagnostic centers; and Enugu State, with 72 Diagnostic centers.
With rising burden of infectious diseases such as malaria, HIV, and tuberculosis, the demand for accurate and timely diagnostic services is surging. The increased availability of diagnostic centers, particularly in urban and semi-urban areas, has improved healthcare accessibility and reduced patient turnaround times, thereby boosting the adoption of IVD technologies. Establishing private labs such as 54gene and Lifebank and public initiatives under the Nigeria Centre for Disease Control (NCDC) has increased testing capacity for COVID-19 and Lassa fever.
These centers often rely on molecular diagnostics, immunoassays, and rapid test kits. Collaborations with international partners have led to capacity-building and technology transfer. Expansion of diagnostic capabilities improves accurate diagnosis, disease monitoring, supporting public health responses, and shaping healthcare policies - all of which contribute to sustained growth in Nigeria’s infectious disease IVD market.
Key players are focusing on boosting local manufacturing capacity, fostering innovation, and reducing dependency on imported diagnostic products. In May 2025, Codix Pharma Ltd, a Nigerian pharmaceutical firm, commissioned Codix Bio Ltd, aIVD manufacturing facility in Sagamu, Ogun State. This facility, the first in Nigeria and the second in sub-Saharan Africa, is expected to produce over 147 million rapid diagnostic test kits annually for malaria, HIV, and hepatitis B and C. This development addresses the urgent need for timely, affordable, locally produced diagnostics, especially for underserved populations. Such initiatives strengthen healthcare independence, improve disease surveillance, and propel market growth.
The comparative company analysis evaluates and categorizes the Nigeria Infectious Disease In-vitro Diagnostics Market based on product portfolio (product satisfaction, product features, and availability), recent market developments (merger & acquisition, new product launch & enhancement, investment & funding, award, agreement, collaboration, & partnership, recognition, and expansion), and geographic presence that aids better decision-making and understanding of the competitive landscape. The report profoundly explores the recent significant developments and innovations by the leading vendors in the Nigeria Infectious Disease In-vitro Diagnostics Market. The key market players are Abbott Laboratories, F. Hoffmann-La Roche Ltd, Becton Dickinson and Co, Sysmex Corp, bioMerieux SA, Bio-Rad Laboratories Inc, QIAGEN NV, QuidelOrtho Corp, Bruker Corp
Based on application, the Nigeria infectious disease in-vitro diagnostics market is segmented into HIV/AIDS, tuberculosis, hepatitis B & C, malaria, others. In 2024, the malaria, segment held the largest share of the market. Based on end user, the Nigeria infectious disease in-vitro diagnostics market is segmented into hospitals and clinics, diagnostic laboratories, blood bank, others. In 2024, the hospitals and clinics segment held the largest share of the market.
Various organic and inorganic strategies are adopted by companies operating in the Nigeria Infectious Disease In-vitro Diagnostics Market. The organic strategies mainly include product launches and product approvals. Inorganic growth strategies witnessed in the market are acquisitions, collaboration, and partnerships. These growth strategies allow the market players to expand their businesses and enhance their geographic presence, along with contributing to the overall market growth. Furthermore, strategies such as acquisitions and partnerships helped strengthen their customer base and extend their product portfolios. A few of the significant developments by key players in the Nigeria Infectious Disease In-vitro Diagnostics Market are listed below.
In April 2025, Bruker Corporation announced significant expansions to its infectious disease testing solutions at the ESCMID Global conference in Vienna, including a broader assay menu for its BeGenius molecular diagnostics system and improvements to its microbial identification platforms.
Reasons to Buy
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the Nigeria Infectious Disease In-vitro Diagnostics Market
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the Nigeria Infectious DiseaseIn-vitro Diagnostics Market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth Nigeria market trends and outlook coupled with the factors driving the market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin security interest with respect to client products, segmentation, pricing and distribution.
Table of Contents
Companies Mentioned
Some of the leading companies in the Nigeria Infectious Disease In-vitro Diagnostics Market include:- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- Becton Dickinson and Co
- Sysmex Corp
- bioMerieux SA
- Bio-Rad Laboratories Inc
- QIAGEN NV
- QuidelOrtho Corp
- Bruker Corp
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 89 |
| Published | June 2025 |
| Forecast Period | 2024 - 2031 |
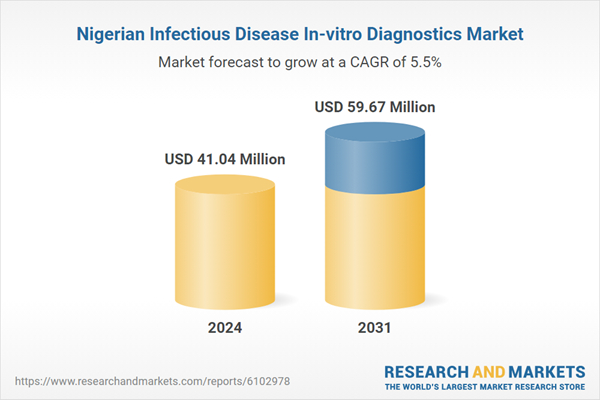
| Estimated Market Value ( USD | $ 41.04 Million |
| Forecasted Market Value ( USD | $ 59.67 Million |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Nigeria |
| No. of Companies Mentioned | 10 |