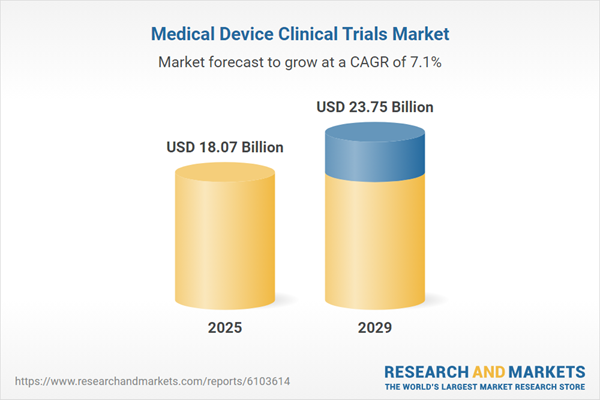
The medical device clinical trials market size is expected to see strong growth in the next few years. It will grow to $23.75 billion in 2029 at a compound annual growth rate (CAGR) of 7.1%. The growth in the forecast period can be attributed to increasing demand for personalized medical devices, rising focus on real-world evidence, growing use of AI in trial design and monitoring, expansion of decentralized and virtual trials, regulatory support for faster approvals, and increasing investments in digital health innovations. Major trends in the forecast period include adoption of decentralized trials, integration of AI and big data analytics, use of wearable monitoring devices, enhanced regulatory harmonization, and advancements in trial design and data analytics.
The forecast of 7.1% growth over the next five years reflects a slight reduction of 0.1% from the previous projection. This reduction is primarily due to the impact of tariffs between the US and other countries. Tariff escalations are likely to burden clinical research organizations by driving up the cost of trial monitoring systems and implantable sensor technologies sourced from the Netherlands and South Korea, exacerbating medical innovation costs and increasing regulatory approval timelines. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The increasing prevalence of chronic diseases is expected to drive the growth of the medical device clinical trials market. Chronic diseases are long-term health conditions that progress slowly and require continuous management. These diseases are on the rise due to sedentary lifestyles, where individuals spend extended hours sitting at desks or using electronic devices, reducing calorie expenditure and weakening cardiovascular health. This leads to conditions such as obesity, diabetes, and hypertension. As chronic diseases become more prevalent, there is a greater need for medical device clinical trials to develop and validate innovative devices for diagnosis, treatment, and management. For example, in June 2023, the Institute for Health Metrics and Evaluation, a U.S.-based public health research institute, reported that over half a billion people worldwide were living with diabetes, and this number is projected to more than double, reaching 1.3 billion people by 2050. Consequently, the rising prevalence of chronic diseases is driving the growth of the medical device clinical trials market.
Companies in the medical device clinical trials market are focusing on AI-powered medical device software to optimize clinical trial protocols and resource allocation, improving trial efficiency and reducing costs. AI-powered medical device software uses artificial intelligence (AI) algorithms, such as machine learning or deep learning, to support or automate medical tasks. For instance, in January 2025, Risklick, a Switzerland-based pharmaceutical company, launched Protocol AI, an AI-based software designed to accelerate and optimize clinical trials for medical devices. Protocol AI uses Natural Language Processing (NLP) and Machine Learning (ML) to analyze clinical data, publications, and regulatory documents, automatically drafting clinical trial protocols in minutes. This innovation significantly reduces the time and cost required for protocol development, which traditionally takes around six months, and has already demonstrated up to a 50% reduction in document development time for medicinal products.
In January 2024, eMed, a U.S.-based health services company, acquired Science 37 for \$38 million. This acquisition strengthens eMed's clinical research capabilities by combining telehealth and diagnostic services with decentralized trial technology to improve patient enrollment and reach underrepresented groups. Science 37 is a U.S.-based research company specializing in decentralized clinical trials, including those for medical devices.
Major players in the medical device clinical trials market are Abbott Laboratories, Siemens Healthineers, Stryker Corporation, Philips Healthcare, Baxter International, Roche Diagnostics, ICON, Intuitive Surgical, Edwards Lifesciences, Fortrea, Medidata, NAMSA, Veranex, TFS HealthScience, Avania, Parexel, Meditrial, Syneos Health, Qserve CRO, Clinius Ltd, Eclevar Medtech, 1med Sa, and ISS AG.
North America was the largest region in the medical device clinical trials market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in medical device clinical trials report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the medical device clinical trials market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The medical device clinical trials market includes revenues earned by entities by providing services such as clinical trial design and management, regulatory consulting, site selection and monitoring, patient recruitment, data management, and statistical analysis. The market value includes the value of related goods sold by the service provider or included within the service offering. The medical device clinical trials market also includes sales of diagnostic devices, therapeutic devices, surgical instruments, monitoring equipment, implantable devices, wearable health technologies, and medical imaging systems. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The medical device clinical trials market research report is one of a series of new reports that provides medical device clinical trials market statistics, including the medical device clinical trials industry global market size, regional shares, competitors with the medical device clinical trials market share, detailed medical device clinical trials market segments, market trends, and opportunities, and any further data you may need to thrive in the medical device clinical trials industry. This medical device clinical trials market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Medical device clinical trials are research studies conducted to evaluate the safety and effectiveness of medical devices in humans. The process includes phases such as feasibility, pivotal, and post-approval studies, which are essential for regulatory approval. These trials generate critical data for device market authorization and continued safety monitoring.
The main study types in medical device clinical trials include feasibility and pilot studies, pivotal studies, FDA premarket approval (PMA) applications, and post-approval studies. Feasibility and pilot studies are early-phase trials with a small group of participants to assess the practicality, safety, and potential challenges of a new device before progressing to larger-scale trials. These studies span various indications such as cardiovascular, neurology, orthopedic, diagnostic imaging, anesthesia and respiratory devices, and others. Study designs typically include interventional, observational, and expanded access categories.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values and are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Medical Device Clinical Trials Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on medical device clinical trials market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for medical device clinical trials? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The medical device clinical trials market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Study Type: Feasibility and Pilot Study; Pivotal Study; FDA Premarket Approval (PMA) Application; Post-Approval Study2) By Indication: Cardiovascular Devices; Neurology Devices; Orthopedic Devices; Diagnostic Imaging; Anesthesia and Respiratory Devices; Other Indications
3) By Study Design: Interventional; Observational; Expanded Access
Subsegments:
1) By Feasibility and Pilot Study: First-in-Human (FIH) Trials; Device Safety Assessment; Procedural Feasibility Studies; Prototype Testing2) By Pivotal Study: Comparative Effectiveness Studies; Randomized Controlled Trials (RCTs); Non-Inferiority Trials; Superiority Trials
3) By FDA PMA (Pre-Market Approval) Application: Clinical Data Submission; Device Risk Analysis; Effectiveness Evidence Studies; Manufacturing Process Validation
4) By Post-Approval Study: Long-Term Safety Monitoring; Real-World Evidence Collection; Registry Studies; Comparative Outcome Studies
Companies Mentioned: Abbott Laboratories; Siemens Healthineers; Stryker Corporation; Philips Healthcare; Baxter International; Roche Diagnostics; ICON; Intuitive Surgical; Edwards Lifesciences; Fortrea; Medidata; NAMSA; Veranex; TFS HealthScience; Avania; Parexel; Meditrial; Syneos Health; Qserve CRO; Clinius Ltd; Eclevar Medtech; 1med Sa; ISS AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Medical Device Clinical Trials market report include:- Abbott Laboratories
- Siemens Healthineers
- Stryker Corporation
- Philips Healthcare
- Baxter International
- Roche Diagnostics
- ICON
- Intuitive Surgical
- Edwards Lifesciences
- Fortrea
- Medidata
- NAMSA
- Veranex
- TFS HealthScience
- Avania
- Parexel
- Meditrial
- Syneos Health
- Qserve CRO
- Clinius Ltd
- Eclevar Medtech
- 1med Sa
- ISS AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 18.07 Billion |
| Forecasted Market Value ( USD | $ 23.75 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |