The increase in chronic illnesses - such as autoimmune diseases, cancer, and infectious disorders - has led to the rising adoption of biologics, which depend heavily on well-characterized cell lines. This has also elevated the need for quality-controlled production systems. Contract development and manufacturing organizations (CDMOs) have contributed significantly to market momentum.
Small and mid-sized biotech firms are increasingly outsourcing their cell line development to CDMOs to reduce overhead costs and accelerate timelines, benefiting from their technical expertise, scalable platforms, and regulatory compliance with agencies like the FDA. This trend allows companies to focus on core competencies such as research and innovation while leveraging CDMOs for end-to-end services including cell line screening, clone selection, process optimization, and GMP manufacturing. These partnerships also offer access to advanced technologies, streamlined workflows, and proven quality assurance frameworks, enabling faster entry into clinical trials and reducing risks associated with internal capacity limitations.
The reagents and media segment generated USD 3.4 billion in 2024. This segment continues to dominate due to its central role in cell growth and maintenance across both R&D and production stages. With a notable increase in the use of mammalian cells for biologics manufacturing, the demand for high-efficiency reagents and cell culture media has surged.
A growing preference for protein-free and serum-free media is helping the segment gain traction, offering improved scalability, reduced risk of contamination, and consistency in large-scale production environments. Such media types are essential for regulatory approval and efficiency in biologics manufacturing, which demands stringent process control.
In terms of cell type, mammalian cells segment generated the highest revenue in 2024, accounting for 71.4%. Their dominance is linked to their superior ability to express human-compatible therapeutic proteins with accurate post-translational modifications. Widely used cell lines such as CHO and HEK-293 are favored in biologics production due to their robust protein folding, glycosylation, and scalability. Technological progress in gene editing tools, such as CRISPR-Cas9, combined with automation and high-throughput systems, continues to enhance the speed and stability of mammalian cell line development, further reinforcing their leadership in the market.
United States Cell Line Development Market was valued at USD 2.6 billion in 2024, driven by a combination of advanced biopharma infrastructure, high rates of chronic disease, and strong adoption of cutting-edge biologic therapies. The country’s well-established pharmaceutical ecosystem, which includes top-tier biotech firms and extensive R&D investment, plays a pivotal role in expanding the market. Research institutions and innovation hubs across the US are also accelerating technological advancements in gene editing and automated platforms, leading to faster and more efficient development cycles. Government and private sector funding are also propelling innovation, further supporting the country’s leadership in the field.
Key companies operating in the Global Cell Line Development Market include PromoCell, Novartis, Genscript Biotech, Sartorius, Thermo Fisher Scientific, ProBioGen, Cytiva (Danaher Corporation), ASIMOV, Advanced Instruments, Sigma Aldrich (Merck KGaA), Eurofins Scientific, WuXi AppTec, Aragen Life Sciences, Fyonibio, and Lonza Group. Top-tier players in the cell line development space are heavily investing in technological innovations like automated cell screening platforms, single-use bioreactors, and CRISPR-based genome editing.
Many companies are forming strategic alliances with biotech firms and CDMOs to enhance service offerings and expand production capabilities. Acquisitions are also a popular move to integrate novel technologies or gain regional access. Firms are focusing on R&D to develop serum-free and chemically defined media, helping improve regulatory compliance and scalability.
Comprehensive Market Analysis and Forecast
- Industry trends, key growth drivers, challenges, future opportunities, and regulatory landscape
- Competitive landscape with Porter’s Five Forces and PESTEL analysis
- Market size, segmentation, and regional forecasts
- In-depth company profiles, business strategies, financial insights, and SWOT analysis
This product will be delivered within 2-4 business days.
Table of Contents
COMPANIES MENTIONED
The companies featured in this cell line development market report include:- Advanced Instruments
- Aragen Life Sciences
- ASIMOV
- Cytiva (Danaher Corporation)
- Eurofins Scientific
- Fyonibio
- Genscript Biotech
- Lonza Group
- Novartis
- ProBioGen
- PromoCell
- Sartorius
- Sigma Aldrich (Merck KGaA)
- Thermo Fisher Scientific
- WuXi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 145 |
| Published | June 2025 |
| Forecast Period | 2024 - 2034 |
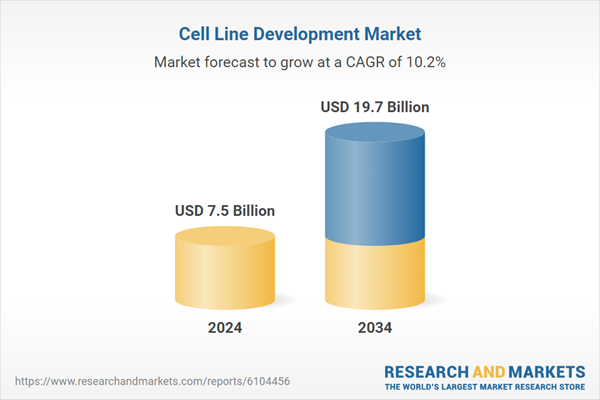
| Estimated Market Value ( USD | $ 7.5 Billion |
| Forecasted Market Value ( USD | $ 19.7 Billion |
| Compound Annual Growth Rate | 10.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |